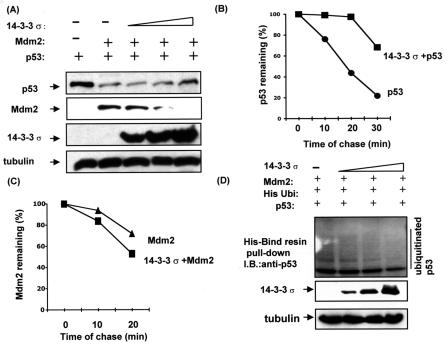
FIG. 3.
14-3-3σ inhibited Mdm2-mediated p53 ubiquitination. (A) 14-3-3σ stabilized p53 in the presence of Mdm2. H1299 cells were cotransfected with indicated pCMV-Mdm2, pCMV-p53, and increasing amounts of pCMV-Flag-14-3-3σ (0 μg, 0 μg, 1 μg, 4 μg, and 8 μg). Equal amounts of protein from cell lysates were immunoblotted with anti-Mdm2, anti-Flag, and antiactin. Levels of tubulin are shown as equal loading controls. (B) 14-3-3σ inhibited p53 turnover. 293T cells transiently transfected with pCMV-14-3-3σ and vector/pCMV-p53 were pulse-labeled with [35S]methionine for 30 min and chased for the indicated time. (C) 14-3-3σ accelerated Mdm2 degradation. 293T cells transiently transfected with pCMV-14-3-3σ and vector/pCMV-Mdm2 were pulse-labeled with [35S]methionine for 30 min and chased for the indicated time. Cells were harvested, and the amount of labeled Mdm2 or p53 protein immunoprecipitated at each time point was detected by phosphoimager for quantitation. p53 or Mdm2 remaining is indicated graphically. (D) 14-3-3σ inhibited Mdm2-mediated p53 ubiquitination. H1299 cells were cotransfected with equal amount of Mdm2 (3 μg), pCMV-p53 (1 μg), histidine-tagged ubiquitin-expressing vector (1 μg), and increasing amount of pCMV-Flag-14-3-3σ (0 μg, 1 μg, 4 μg, and 8 μg). The cell lysates were harvested, and the histidine-tagged ubiquitin-containing protein complexes were pulled down with His-Bind resin (Novagen). The protein complexes were then resolved by SDS-12% polyacrylamide gel and probed with anti-p53 antibody to observe the histidine-tagged ubiquitinated p53.