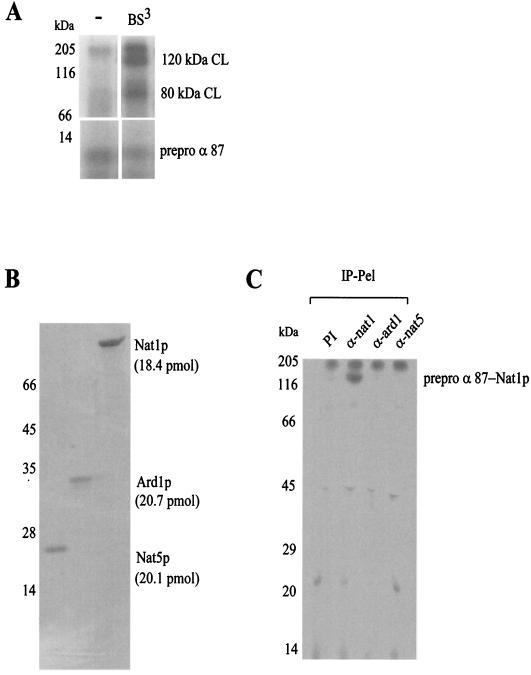
FIG. 1.
Nat1p, a subunit of yeast NatA interacts with RNCs. (A) RNCs carrying 87 N-terminal amino acids of 35S-labeled prepro α-factor (prepro α 87) were incubated in the absence (−) or presence of the homobifunctional cross-linker BS3 as indicated. Aliquots were run on 10% Tris-Tricine gels and were subsequently analyzed by autoradiography. The amounts of prepro α 87 and the cross-link products (CL) were quantified by densitometry to approximate the cross-linking efficiency. The upper panel (120 kDa CL, 80 kDa CL) shows a four-times-longer exposure than the lower panel (prepro α 87); the signal was linear in relation to the exposure time. The amount of prepro α 87 was set to 100%. (B) Subunits of the cross-link-inducing complex were separated by reverse-phase HPLC and analyzed on a Coomassie-stained gel. The amount of protein contained in the different fractions was determined by using the peak area of the HPLC profile and the calculated molar extinction coefficients (see Materials and Methods). (C) Cross-link reactions were performed with low-salt treated RNCs carrying 35S-labeled prepro α 87 as a nascent chain. Aliquots of the cross-link reactions were subjected to immunoprecipitation under denaturing conditions with preimmune serum (PI), anti-nat1 (α-nat1), anti-ard1 (α-ard1), and anti-nat5 (α-nat5) antibodies. Shown is the material bound to the respective antibody (IP-Pel). Immunoprecipitation of the 120-kDa cross-link with anti-nat1 was quantitative (data not shown).