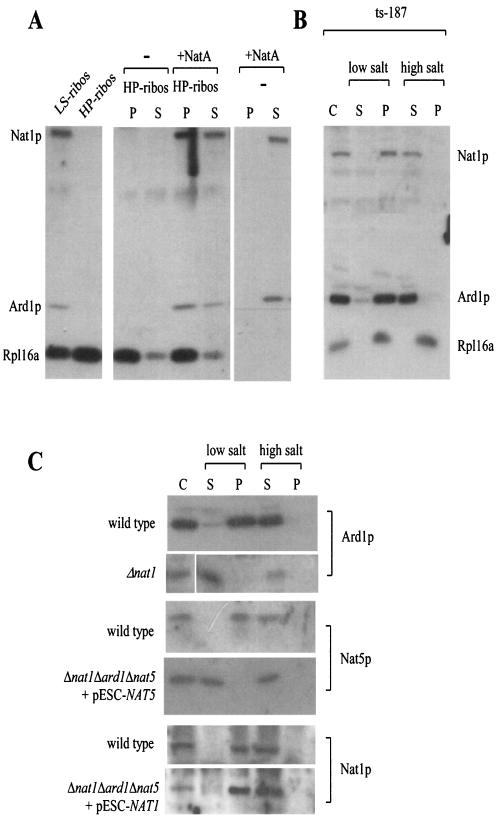
FIG. 3.
Nat1p anchors Ard1p and Nat5p to the ribosome. (A) Nascent chain-free ribosomes, generated by high-salt-puromycin treatment, were isolated, resuspended, incubated in the absence (−) or presence (+NatA) of partially purified NatA, and subsequently reisolated by centrifugation. As a control, NatA was subjected to centrifugation in the absence of ribosomes. LS-ribos, ribosomes isolated at low-salt conditions; HP-ribos, high-salt-puromycin treated ribosomes; P, ribosomal pellet; S, postribosomal supernatant. (B) The yeast strain ts-187, defective in translationinitiation at elevated temperatures, was incubated at 37°C for 20 min prior to harvest. Subsequently, cytosol (C) was prepared as described in Materials and Methods and separated into the ribosomal pellet (P) and a postribosomal supernatant (S) in the presence of low salt (120 mM potassium acetate) or high salt (700 mM potassium acetate) concentrations. (C) Cytosol (C) from a wild-type yeast strain, a strain lacking Nat1p (Δnat1), a strain lacking Ard1p and Nat1p but expressing NAT5 under control of a GAL promoter (Δnat1Δard1Δnat5 + pESC-NAT5), and a strain lacking Nat5p and Ard1p but expressing NAT1 under control of a GAL promoter (Δnat1Δard1Δnat5 + pESC-NAT1) was separated into a postribosomal supernatant (S) and a ribosomal pellet (P) in the presence of low or high salt concentrations as described above. (A to C) Samples were separated on 10% Tris-Tricine gels, transferred onto nitrocellulose and decorated with antibodies directed against Nat1p, Ard1p, Nat5p, and Rpl16a (ribosomal marker) as indicated.