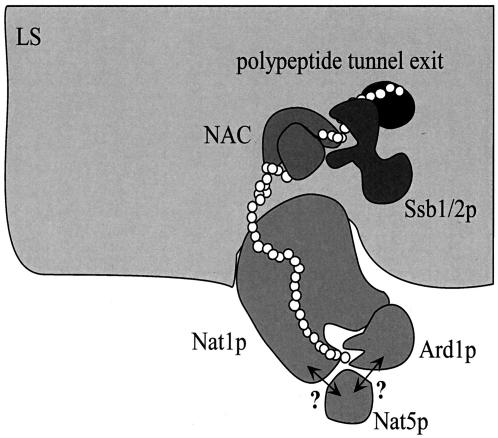
FIG. 7.
A model for cotranslational Nα-acetylation of polypeptides by NatA. Nat1p, the noncatalytic NatA subunit, mediates the stable interaction of the complex with the large ribosomal subunit (LS). In addition, Nat1p contacts the nascent polypeptide when approximately 40 amino acids have emerged from the exit tunnel (i.e., the nascent chain has a total length of approximately 80 amino acids). This interaction, possibly mediated via TPRs contained in Nat1p, may guide the growing polypeptide to the catalytic subunit Ard1p and the putatively catalytic subunit Nat5p, which transfer an acetyl moiety from acetyl-coenzyme A to specific N-terminal amino acids (see the introduction). Ard1p requires the presence of Nat1p for stable association with the ribosome. It is unknown whether Nat5p interacts with the ribosome via Nat1p, Ard1p, or both (indicated by question marks). The catalytic subunits may have little or no affinity for the nascent polypeptide in the absence of Nat1p; hence, a stable complex with Nat1p is essential for Nα-acetyltransferase activity. Complex formation between Nat1p and Ard1p might occur via predicted coiled coil domains in their C-terminal regions. Two other ribosome-bound factors, NAC and the Hsp70 homolog Ssb1/2p bind to nascent chains between 40 and 50 amino acids in length. Binding of NAC and Ssb1/2p precedes the binding of Nat1p. Whether or not NAC, Ssb1/2p, and NatA simultaneously bind to one and the same ribosome (as suggested here) or independently to different ribosome molecules is currently unknown. For details, see Results and Discussion. Please note that other factors bound to the ribosome or the nascent chain have been omitted for simplicity.