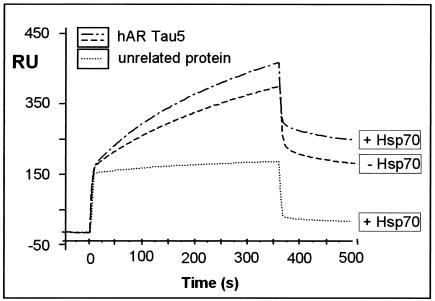
FIG. 4.
Hsp70 enhances the interaction of Bag-1L with the τ5 domain of the AR. A representative sensogram is presented, showing the interaction of Bag-1L (100 ng/μl) with the τ5 domain of the AR (1,500 RU) coupled to the surface of a CM5 sensor chip. The interaction data were obtained in the absence and presence of Hsp70 (100 ng/μl) and compared to the use of an unrelated protein (recombinant GST; 100 ng/μl). The values for the apparent rate constants for the interaction of Bag-1L with τ5 of the AR are as follows: Ka, 1.41 × 103 M−1s−1; Kd, 8.54 × 10−4 s−1. From these data, the apparent dissociation equilibrium constant was calculated to be KD = 6.07 × 10−7 M. All calculations were based on the assumption of monomolecular 1:1 binding characteristics.