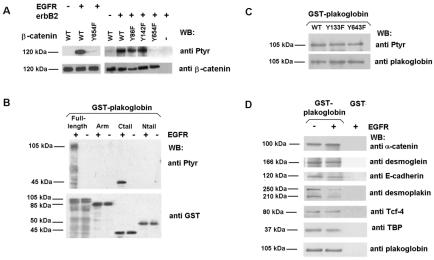
FIG. 2.
EGFR presents different substrate specificities of phosphorylation on β-catenin and plakoglobin. (A) GST-β-catenin fusion proteins (6.7 pmol) were phosphorylated with 0.5 U of recombinant EGFR kinase or RWP1 cell extracts transfected with erbB2. Phosphorylation was analyzed by Western blotting (WB) with anti-PTyr MAb. The membrane was stripped and reprobed for β-catenin as a control, and similar levels of GST-β-catenin were present. (B and C) Five picomoles of GST-plakoglobin deletion mutants (B) or point mutants (C) was phosphorylated with EGFR under the indicated conditions. Samples were analyzed by Western blotting with anti-PTyr MAb and reblotted against GST (B) or plakoglobin (C). (D) Five picomoles of GST or GST-plakoglobin fusion proteins was phosphorylated with EGFR as described above. Pull-down assays were then performed, and the GST proteins were incubated with the indicated amounts of total cell extracts from SW480. The associated proteins were detected with specific MAbs. WT, wild type; +, present; −, absent. The estimated molecular masses of the bands detected with each antibody are indicated.