Abstract
Myostatin, a transforming growth factor β (TGF-β) family member, is a potent negative regulator of skeletal muscle growth. In this study we characterized the myostatin signal transduction pathway and examined its effect on bone morphogenetic protein (BMP)-induced adipogenesis. While both BMP7 and BMP2 activated transcription from the BMP-responsive I-BRE-Lux reporter and induced adipogenic differentiation, myostatin inhibited BMP7- but not BMP2-mediated responses. To dissect the molecular mechanism of this antagonism, we characterized the myostatin signal transduction pathway. We showed that myostatin binds the type II Ser/Thr kinase receptor. ActRIIB, and then partners with a type I receptor, either activin receptor-like kinase 4 (ALK4 or ActRIB) or ALK5 (TβRI), to induce phosphorylation of Smad2/Smad3 and activate a TGF-β-like signaling pathway. We demonstrated that myostatin prevents BMP7 but not BMP2 binding to its receptors and that BMP7-induced heteromeric receptor complex formation is blocked by competition for the common type II receptor, ActRIIB. Thus, our results reveal a strikingly specific antagonism of BMP7-mediated processes by myostatin and suggest that myostatin is an important regulator of adipogenesis.
Mesenchymal stem cell differentiation is generally thought to be initiated by the inductive action of specific growth factors, and abundant evidence demonstrates that transforming growth factor β (TGF-β) superfamily members can profoundly regulate these processes (12, 17, 18, 34, 58). For instance, TGF-β can inhibit adipogenesis and myogenesis while bone morphogenetic proteins (BMPs) can promote chondrogenesis, osteogenesis, and adipogenesis. Myostatin (previously known as growth and differentiation factor 8 [GDF8]) is a key critical regulator of skeletal muscle development (26). Myostatin-null mice display widespread increases in muscle mass (36) and decreased body fat accumulation (28, 38), and inhibition of myostatin with blocking antibodies increases muscle mass (8). Myostatin function appears to be well conserved, since mutations in the myostatin gene have been identified in the double-muscled Belgium Blue and Piedmontese cattle breeds (37). Consistent with this, myostatin mRNA is first expressed in somites, in the myotome layer that gives rise to skeletal muscle (36), and is highly expressed in skeletal muscle at later developmental stages and in adults and has been detected in both fetal and adult heart and in adipose tissue (36, 50). Of note, systemic administration of myostatin to adult mice results in a cachexia-like syndrome that is associated with a profound loss of both muscle and fat (64). Since decreased fat accumulation is observed both in knock-out mice that lack myostatin and in wild-type adult mice in which myostatin has been systemically administered (28, 38), it appears that myostatin may play distinct physiological roles during early development and during adult homeostatic processes.
Like all TGF-β superfamily members, myostatin is synthesized in a precursor form that is proteolytically cleaved to release a C-terminal mature ligand (34). Within this mature region, myostatin is most closely related to mammalian GDF11/BMP11 (90% identity), Drosophila myoglianin, and Caenorhabditis elegans unc-129 (11, 31, 35). Interestingly, the pro-domain can antagonize the biological activity of the mature ligand and overexpression of this protein in transgenic mice results in increased muscle mass (54, 60).
TGF-β superfamily members signal through heteromeric receptor complexes composed of two homodimers each of type I and type II serine/threonine kinase receptors (33). A large body of evidence indicates that certain TGF-β superfamily members, including TGF-β, activins, and BMP7, initiate signaling by first directly binding to the type II receptor, which leads to the recruitment of an appropriate type I receptor. A variation on this theme is observed for BMP2 or BMP4 and the highly related Drosophila ligand, decapentaplegic (dpp). Here, the ligand can bind directly to the type I receptor but formation of a high-affinity receptor complex requires the presence of a type II receptor (9, 23, 24, 30, 32, 41, 45, 49, 53, 59). While the TGF-β receptors TβRII and TβRI (also known as ALK5) are currently thought to be specific for TGF-β, other Ser/Thr kinase receptors display more promiscuous behavior. For instance, the type II receptors ActRII and ActRIIB can associate with the type I receptor ALK4 to mediate activin or nodal signals, with ALK7 to mediate nodal signals, and with ALK2, ALK3, or ALK6 to propagate BMP signals (33, 47, 61). In all cases thus far examined, once a receptor complex is formed, the type II receptor phosphorylates the type I receptor in the highly conserved juxtamembrane region known as the GS domain. This activated type I receptor then propagates the signal by phosphorylating members of the Smad family of intracellular mediators (5, 33, 40).
The specificity of biological responses is determined by the type I receptor, which targets specific Smad proteins and thereby initiates distinct intracellular signaling cascades (5, 33, 40). For instance, TβRI (ALK5), ALK4, and ALK7 phosphorylate Smad2 and Smad3 and thereby transduce TGF-β-like signals for TGF-βs, activins, and nodals. In contrast, BMP-like ligands, such as BMP2, BMP4, BMP7, and GDF5 activate the type I receptors ALK2, ALK3, and/or ALK6, which phosphorylate Smad1, Smad5, and Smad8 and thereby generate BMP-specific responses. Once phosphorylated, these receptor-regulated Smads (R-Smads) associate with a common Smad, Smad4. The R-Smad/Smad4 complex then translocates to the nucleus, associates with one of many potential DNA-binding partners and thereby positively or negatively regulates the transcription of target genes (5, 33, 40).
TGF-β-like and BMP-like ligands utilize distinct pathways to mediate their biological effects, and growing evidence indicates that these two pathways can antagonize each other's activities (5, 33, 40, 55). BMP7 and BMP2 can induce adipogenesis, and here we demonstrate that myostatin can potently antagonize BMP7- but not BMP2-induced differentiation. To decipher the mechanism underlying the biological role of myostatin, we have identified the myostatin receptors and its intracellular signaling pathway. We demonstrate that myostatin efficiently binds the type II receptor ActRIIB and forms a heteromeric complex with the type I receptor ALK4 or ALK5, thereby inducing phosphorylation of Smad2 and Smad3 to activate a TGF-β-like signaling pathway. This represents the first demonstration that ALK5 can mediate signals for a ligand other than TGF-β. Furthermore, we reveal that myostatin specifically antagonizes BMP7 but not BMP2 by competing for binding to the type II receptor. These findings suggest that differential antagonism of BMPs by myostatin may be an important mechanism underlying the control of mesenchymal cell differentiation in development and homeostasis.
MATERIALS AND METHODS
Cell culture and differentiation assays.
C3H 10T1/2 and 3T3-L1 cells were obtained from the American Type Culture Collection (Manassas, Va.). For differentiation assays, C3H 10T1/2 (between passages 10 and 15) and 3T3-L1 cells were plated at 2,000 cells/cm2 in Dulbecco's modified Eagle's medium containing high glucose supplemented with 10% fetal bovine serum (FBS) and antibiotics on day 0. Growth medium was replaced on day 2 with medium containing 10% FBS and combinations of myostatin, BMP7, or BMP2, and cells were cultured for an additional 6 to 10 days. Lipid accumulation was visualized with the Oil Red O stain (Sigma) as described previously (43). Briefly, cell monolayers were washed once with cold phosphate-buffered saline, fixed for 1 h in 10% neutral formalin buffer, and stained for 1 h in Oil Red O solution. Stained cells were washed briefly with 70% ethanol, and excess stain was removed by washing in water.
Transcriptional activation and RNA interference assays.
C3H 10T1/2, 3T3-L1, and HepG2 cells were transiently transfected by using Lipofectamine (Life Technologies, Gaithersburg, Md.), Superfect (Qiagen, Valencia, Calif.), or the calcium phosphate-precipitation method, respectively (19). RIB L17 and DR 27 cells were transfected by the DEAE-dextran method as described previously (4). The next day, the cells were washed with medium containing 0.2% FBS and treated overnight with the indicated ligands. Cell lysates were harvested 48 h posttransfection, and luciferase activity was normalized to β-galactosidase activity. The 3TP-Lux, A3-Lux, SBE4-Lux, ALK4, ALK5, and TβRII constructs were described previously (16, 57, 62). Small interfering RNAs were purchased as single-strand oligonucleotides, deprotected, and annealed as specified by the manufacturer (Dharmacon Research Inc., Lafayette, Colo.). The oligonucleotide sequences for the ActRIIB-specific siRNA are 5′AACTTCTGCAACGAGCGCTTC3′ and 5′AAGAAGCGCUCGUUGCAGAAG3′, and the irrelevant scrambled siRNAs are 5′ AAGGGCAAGACGAGCGGGAAG3′ and 5′AACUUCCCGCUCGUCUUGCCC3′.
Smad phosphorylation.
Subconfluent cell monolayers were preincubated in low-serum medium for 3 h and then treated with ligand for 1 h. The cells were washed once with cold phosphate-buffered saline and lysed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1 mM dithiothreitol, 10% [vol/vol] glycerol) containing phosphatase and protease inhibitors as described previously (19). Cell lysates were either immunoprecipitated with anti-Smad2/Smad3 goat polyclonal antibody (Upstate Biotechnology Inc., Lake Placid, N.Y.) or directly separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and subjected to immunoblotting with anti-phospho-Smad1, anti-phospho-Smad2 (Upstate Biotechnology), or anti-phospho Smad2/Smad3 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.). Total Smad1, Smad2, and Smad3 levels were detected with rabbit anti-Smad1 (32), mouse anti-Smad2 (Transduction Laboratories, Lexington, Ky.), or rabbit anti-Smad3 (Zymed Laboratories, San Francisco, Calif.) antibodies, and proteins were visualized by chemiluminescence.
RNA isolation and PCR analysis.
Total cellular RNA was extracted from cell monolayers with Trizol reagent (Invitrogen, Carlsbad, Calif.). First-strand cDNA was reverse transcribed from 2 μg of total RNA with either the 3′ specific primer or random hexanucleotides with RevertAid H Minus Moloney murine leukemia virus M-MuLV reverse transcriptase (MBI Fermentas, Hanover, Md.) as specified by the manufacturer. Primer sequences and conditions for PCR were as described previously for peroxisome proliferator-activated receptor γ2 (PPARγ2), CCAAT enhancer binding protein α (C/EBPα), glyceraldehyde 3-phosphate dehydrogen (GPDH) (15), lipoprotein lipase (LPL) (25), adipose tissue-specific secretory factor (22), and hypoxanthine phosphoribosyltransferase (HPRT) (52). PCR was performed within the linear range of amplification for each primer pair.
For quantitative PCR (Q-PCR) of ActRIIB transcripts, cDNA was reverse transcribed from DNase I-treated RNA and amplified using Brilliant SYBR Green Q-PCR master mix as specified by the manufacturer (Stratagene, Cedar Creek, Tex.), using an ABI Prism 7700 sequence detection system (Applied Biosystems, Inc., Foster City, Calif.). Oligonucleotide primers were designed using Primer Express software (Applied Biosystems, Inc.) and were as follows: ActRIIB, 5′CCCCCACCTTCCCCATTAC3′ and 5′CCAAATCTTCCCCTTGCTTTC3′; ActRII, 5′CCCATGGGCAGGTTGGTA3′ and 5′ATGCGTCCCTTTGGAAGTTTATAG3′; and HPRT, 5′AAACAATGCAAACTTGCTTTCC3′ and 5′GGTCCTTTTCACCAGCAAGCT3′. The oligonucleotides were validated to amplify the desired target within a linear range of template cDNA concentrations. Data from triplicate cDNA standards were exported from the ABI Prism 7700 SDS software into a Microsoft Excel spreadsheet, and the Trendline option was utilized to construct a relative standard curve. The input amount of ActRIIB or ActRII transcript in the test samples was calculated with the equation of the standard curve and normalized to the amount of HPRT per total RNA in each sample. All samples were assayed for ActRIIB, ActRII, and HPRT in triplicate in two independent experiments.
Affinity labeling.
Receptor constructs as described previously (4, 19, 57) were transiently transfected into COS-1 cells by the DEAE-dextran method. Purified myostatin was labeled with 125I as described previously (54). COS-1 cells were affinity labeled with 1 ng of [125I]myostatin per ml with or without 500 ng of unlabeled myostatin per ml for 4 h at 4°C, the receptors were cross-linked to the ligand with BS3 reagent (Pierce), and cells were lysed as described previously (54). Total-cell lysates were separated by SDS-PAGE, and 125I-bound ligand was visualized by autoradiography. For BMPs, transfected COS-1 cells were affinity labeled with 2 nM [125I]BMP7 or 2 nM [125I]BMP4 and cross-linked with disuccinimidyl suberate (Pierce, Rockford, Ill.) as described previously (57). Lysates were subjected to immunoprecipitation with anti-HA monoclonal antibodies (12CA5; Roche Diagnostics), and receptor-ligand complexes were visualized by SDS-PAGE and either autoradiography or phosphorimaging.
RESULTS
Myostatin inhibits mesenchymal cell differentiation to adipocytes.
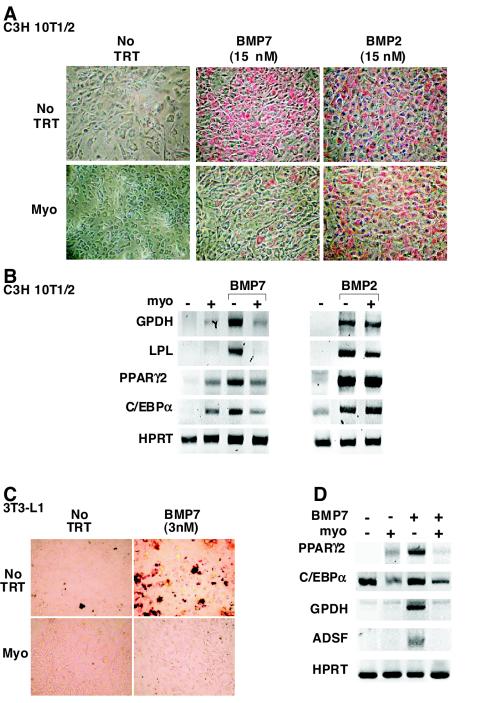
BMPs play an important role in the determination and differentiation of mesenchymal progenitors along a variety of lineages, including that of adipogenic pathways (58). For instance, both BMP2 and BMP7 can induce adipogenic conversion of the pluripotent mesenchymal precursor cells, C3H 10T1/2 (1, 3, 56). Since exogenous myostatin can induce fat loss in vivo (28, 64), we examined the effect of myostatin on C3H 10T1/2 differentiation in the presence and absence of BMP. We first confirmed by reverse transcription-PCR analysis that undifferentiated cells express all of the relevant receptors including the type II receptors TβRII, ActRII, ActRIIB, and BMPRII and the type I receptors, ALK2, ALK3, ALK4, and ALK5, although they did not express ALK6 (data not shown). Prior to ligand addition, C3H 10T1/2 cells are nonrefractile and resemble fibroblasts in morphology. After 10 days of culture, untreated or myostatin-treated cells adopted a polygonal shape but did not accumulate lipid (Fig. 1A). However, treatment of cells with BMP2 or BMP7 induced adipogenic conversion of the cells as indicated by the presence of round, red, lipid-filled cells on Oil Red O staining (Fig. 1A). The effect of myostatin on BMP-induced adipogenesis was then examined. Myostatin blocked the adipogenic effect of BMP7 but had no effect on BMP2-induced differentiation (Fig. 1A). In parallel, we evaluated the expression of adipogenesis-induced genes including those encoding the late markers GPDH and LPL and the transcription factors PPARγ2 and C/EBPα (13, 48). Treatment of cells with BMP7 or BMP2 induced expression of all four markers. Consistent with the Oil Red O staining, the BMP7-induced increase in the expression of the late markers was blocked by coincubation with myostatin (Fig. 1B). BMP7-induced expression of the transcription factors was also reduced by myostatin. However, we noted that myostatin alone was capable of inducing a low level of PPARγ2 and C/EBPα expression, although this low level was not sufficient to promote adipogenesis as assessed by Oil Red staining (Fig. 1A). In contrast to BMP7, myostatin had no effect on the BMP2-induced expression of the adipogenic markers.
FIG. 1.
Myostatin blocks BMP7-induced adipocyte differentiation. C3H 10T1/2 (A and B) and 3T3-L1 (C and D) cells were incubated for 10 days in the presence of various combinations of BMP2, BMP7, myostatin (10 nM), or no ligand (no TRT) as indicated. (A and C) Lipid accumulation was assessed in fixed cells by Oil Red O staining. (B and D) The effect of myostatin on the BMP2 (3 nM)- and BMP7 (3 nM)-regulated expression of key adipocyte transcription factors and late adipocyte markers was determined by reverse transcription-PCR analysis.
To determine whether myostatin could also block differentiation in other cell lines, we next utilized a preadipocyte cell line, mouse 3T3-L1 cells, and determined whether BMP7 could induce adipogenesis in these cells. 3T3-L1 cells treated with BMP7 for 10 days changed from a nonrefractile, fibroblast-like morphology to a more rounded phenotype and accumulated lipid droplets, as visualized by Oil Red O staining (Fig. 1C). Furthermore, myostatin efficiently blocked BMP7-induced accumulation of lipids and, consistent with this, prevented the induction of both PPARγ2 and C/EBPα as well as the late adipocyte differentiation markers (GPDH and ADSF/resistin) (Fig. 1D). BMP2 also induced adipogenic differentiation of 3T3-L1 cells; however, myostatin was unable to potently antagonize this effect (data not shown).
These results show that myostatin can block BMP7-induced adipogenesis in both mesenchymal precursor cells and preadipocytes whereas it has little effect on BMP2-induced differentiation, suggesting that myostatin is a potent antagonist of BMP7- but not BMP2-induced differentiation.
Myostatin signals through a TGF-β-like pathway.
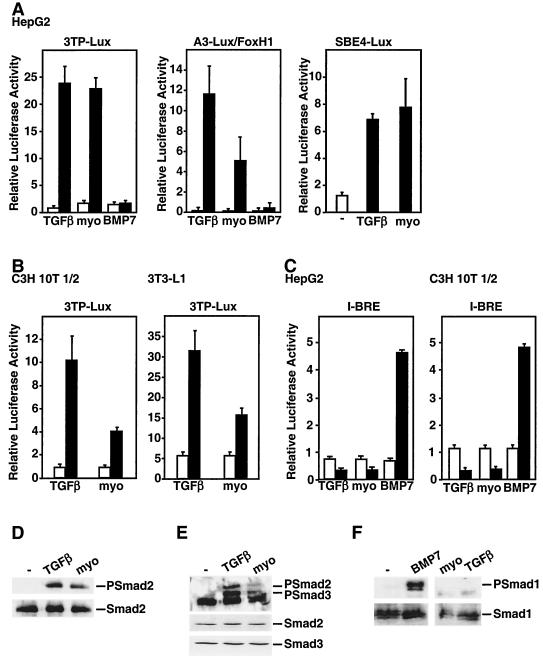
TGF-β superfamily members signal through one of two distinct pathways (5, 33, 40). TGF-β, activin- and nodal-related ligands induce the phosphorylation of Smad2 and Smad3 and thereby activate Smad2/Smad3-dependent target genes. In contrast, BMP/GDF-like ligands induce the phosphorylation of Smad1, Smad5, and/or Smad8 and thereby control expression of specific BMP target genes. Thus, to identify the signaling pathway utilized by myostatin, we first determined whether myostatin preferentially activated TGF-β- or BMP-dependent promoters. For the TGF-β signaling pathway, we used three luciferase reporter constructs: 3TP-Lux, which contains a portion of the PAI-1 promoter (57), A3-Lux, which is composed of three FoxH1 binding sites derived from the Xenopus Mix.2 gene (10), and SBE4-Lux, which contains four copies of an 8-bp palindromic Smad3/Smad4 binding element (62). As described previously, TGF-β treatment of HepG2 cells induced luciferase activity from 3TP-Lux, A3-Lux, and SBE4-Lux (16) whereas BMP7 treatment had no effect (Fig. 2A). Similar to the TGF-β-treated cells, addition of myostatin yielded a ligand-dependent activation of the 3TP-Lux, A3-Lux and SBE4-Lux reporters. Myostatin-dependent activation of TGF-β promoters was also observed in C3H 10T1/2, 3T3-LI, L6E9, C2C12 (Fig. 2B and data not shown), and A204 rhabdomyosarcoma cells (54).
FIG. 2.
Myostatin signals through a TGF-β/activin signaling pathway. (A to C) Myostatin (10 nM) activates the TGF-β-responsive reporters 3TP-Lux, A3-Lux, and SBE4-Lux but not the BMP-responsive reporter I-BRE-Lux. HepG2 (A and C), C3H 10T1/2 (B and C), and 3T3-L1 (B) cells were transiently transfected with the indicated reporter constructs, and luciferase activity in cells treated with TGF-β, myostatin, or BMP7 or left untreated was determined. (D to F). Myostatin (10 nM) induces phosphorylation of endogenous Smad2 and Smad3. 3T3-L1 cells were incubated with TGF-β, myostatin, or BMP7 for 30 min, and cell lysates were subjected to anti-Smad2/Smad3 (D and E) or anti-Smad1 (F) immunoprecipitation followed by immunoblotting with anti-phosphospecific Smad2 (PSmad2), anti-phosphospecific Smad2/Smad3 (PSmad2/3), or anti-phosphospecific Smad1 (PSmad 1) antibodies. Total Smad proteins were detected by anti-Smad2 (D and E), anti-Smad3 (E), and anti-Smad1 (F) antibodies (lower panels).
To determine whether myostatin might also mediate BMP-like signals, we used a BMP-inducible promoter, I-BRE, which contains a portion of intron 1 of the Smad7 gene (7). As expected, BMP7 was able to activate luciferase activity from the I-BRE-Lux reporter in HepG2, C3H 10T1/2 (Fig. 2C), and C2C12 (data not shown) cells. In contrast neither myostatin nor TGF-β induced I-BRE-Lux luciferase activity; moreover, both ligands reduced the basal activity of the reporter (Fig. 2C).
We next determined which Smad was activated in response to myostatin by determining the phosphorylation status of endogenous Smads. For this, C3H 10T1/2 cells were incubated for 1 h with various combinations of TGF-β, myostatin, BMP2, or BMP7. Receptor-dependent phosphorylation on the carboxy-terminal serines of the Smads was then assessed by immunoblotting with anti-phospho-Smad2/Smad3 and anti-phospho-Smad1 antibodies (Fig. 2D to F). As expected, TGF-β induced Smad2 and Smad3 phosphorylation whereas BMP7 induced Smad1 phosphorylation. Similarly to TGF-β, addition of myostatin resulted in phosphorylation of Smad2 and Smad3 but not of Smad1 (Fig. 2D to F). Together, these results indicate that myostatin activates an intracellular signaling pathway that leads to phosphorylation of Smad2 and Smad3 and activation of TGFβ/activin-dependent target genes.
Identification of myostatin receptors.
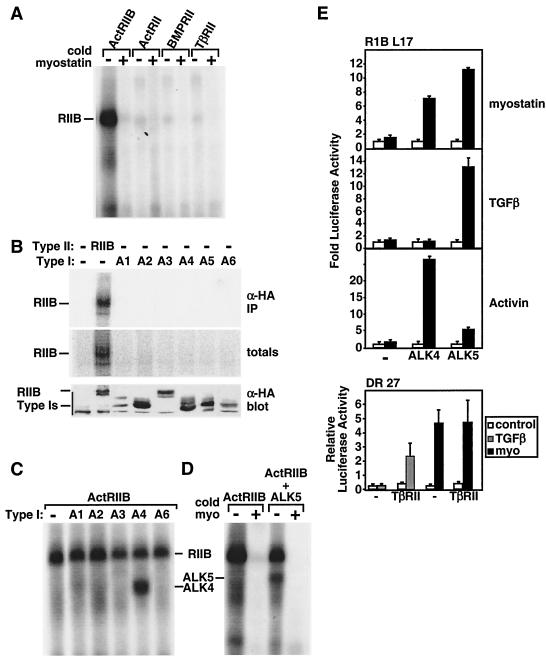
TGF-β superfamily ligands signal through heteromeric complexes of type II and type I Ser/Thr kinase receptors (5, 33, 40). For instance, TGF-β signals are mediated by the type II-type I receptor pair TβRII and TβRI (also known as ALK5). However, unlike TGF-β receptors, other Ser/Thr kinase receptors are much more promiscuous. For example, the type II receptors ActRII and ActRIIB can associate with multiple type I receptors (ALK2, ALK3, ALK4, ALK6, and ALK7) to mediate signals for activins, nodals, and BMPs. Therefore, we next focused on identifying the cognate type I and type II receptors for myostatin. For this, COS-1 cells were transiently transfected with known TGF-β superfamily receptors and [125I]myostatin binding to transfected cells was determined by affinity labeling. We first investigated the binding of [125I]myostatin to cells expressing the type II receptors ActRII, ActRIIB, BMPRII, and TβRII. These results revealed that myostatin specifically bound to ActRIIB but not to ActRII, TβRII, or BMPRII (Fig. 3A). The specificity of binding was confirmed by competition with excess unlabeled myostatin (Fig. 3A). These findings are consistent with recent reports indicating that myostatin and the highly related GDF11 can bind ActRIIB (27). In addition, we observed the previously reported (27) weak binding of [125I]myostatin to ActRII; however, this was detected only when ActRII was expressed at levels 10-fold higher than those of ActRIIB in transfected cells (data not shown). Thus, unlike activin and BMP7 (4, 32), myostatin displays a distinct ligand binding preference for ActRIIB and ActRII.
FIG. 3.
Myostatin binds to ActRIIB and signals through a heteromeric receptor complex composed of either ALK4 or ALK5. (A to D) COS-1 cells were transiently transfected with various type II receptors (A) or individual type I receptors (B) or the type II receptor ActRIIB together with the type I receptors ALK1 to ALK6 (A1 to A6) (C and D) as indicated. The cells were incubated with [125I]myostatin (0.03 nM [A and C] or 1 nM [B]) in the presence or absence of unlabeled myostatin (15 nM). Total cell lysates were separated by SDS-PAGE, and 125I was detected by autoradiography (A, C, and D) or phosphorimaging (B). Expression of receptor protein in total cell lysates was determined by immunoblotting with anti-HA antibody (B). (E) Mv1Lu cell derivatives, RIB L17 or DR 27 cells, were transiently transfected with the 3TP-Lux reporter either with or without the type I receptors ALK4 or ALK5 (upper panel) or the type II receptor TβRII (lower panel). The fold induction of luciferase activity in cells treated with myostatin, TGF-β, or activin relative to controls was determined.
Since BMP2 can directly bind type I receptors, we next tested the ability of [125I]myostatin to bind the type I receptors ALK1 to ALK6. Myostatin binding to ActRIIB was detected in both total cell lysates and immunoprecipitates; however, no binding to any type I receptor was observed (Fig. 3B). Since ligand binding to the type I receptor is often dependent on the presence of a type II receptor and since myostatin appears to bind most efficiently to ActRIIB, we directed further studies toward investigating receptor combinations that included ActRIIB. For this, COS-1 cells were transiently transfected with ActRIIB in combination with the type I receptors ALK1 to ALK6. Interestingly, myostatin receptor complexes affinity labeled with [125I]myostatin were detected in cells coexpressing ActRIIB and either ALK4 (ActRIB [Fig. 3C]) or ALK5 (TβRI [Fig. 3D]) but not in cells expressing ActRIIB with any of the other type I receptors (Fig. 3C). Together, these results reveal that myostatin can bind to ActRIIB alone and to the type I receptors ALK4 and ALK5 when these receptors are coexpressed with ActRIIB. Although it has been previously shown that multiple ligands such as BMPs and activin can bind to ActRIIB and that ALK4 can bind to activin and nodal, no alternative ligand for the TGF-β type I receptor ALK5 has previously been identified (33, 61).
Myostatin signaling is mediated by ALK4 and ALK5.
To assess the ability of ALK4 and ALK5 to mediate myostatin signals we next tested for ligand responsiveness in a TGF-β-resistant derivative of Mv1Lu cells, RIB L17, that lacks the TGF-β type I receptor ALK5 (4). We note that this cell line is also insensitive to activin due to extremely low levels of the activin type I receptor ALK4 (4). Treatment of cells with TGF-β or activin failed to activate 3TP-Lux reporter activity in the absence of coexpressed type I receptors (Fig. 3E). However, transient coexpression of ALK5 restored TGF-β- but not activin-dependent activation of luciferase activity whereas cotransfection of ALK4 restored activin but not TGF-β signaling (Fig. 3E), consistent with the known binding specificity of these type I receptors. Addition of myostatin also failed to activate the 3TP-Lux reporter in control cells; however, coexpression of either ALK4 or ALK5 resulted in myostatin-dependent activation of 3TP-Lux reporter activity (Fig. 3E). Unlike for RIB L17 cells, myostatin signaling was not impaired in the activin-sensitive but TGF-β-resistant Mv1Lu derivative, DR 27, which lacks the TGF-β type II receptor but expresses normal type I receptors (Fig. 3E). Furthermore, coexpression of TβRII restored TGF-β responsiveness to these cells but had no effect on myostatin-dependent signaling. Together with the ligand binding assays, our results reveal that myostatin signals through heteromeric receptor complexes composed of ActRIIB together with either ALK4 or ALK5 to induce Smad2 and Smad3 phosphorylation and activate a TGF-β-like signaling pathway.
Myostatin blocks BMP7- but not BMP2-induced transcriptional responses.
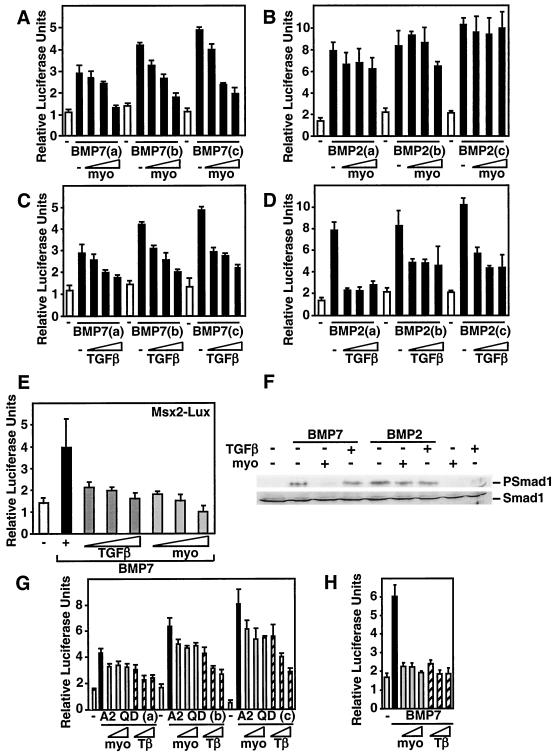
To gain insights into the mechanism through which myostatin antagonizes BMP7-induced differentiation, we analyzed various events in the BMP-dependent signaling cascade. First, we tested whether myostatin abrogates BMP-induced transcriptional activation of the luciferase reporter I-BRE-Lux in C3H 10T1/2 cells. Cells were treated with different doses of BMP7 or BMP2, and the effect of increasing doses of myostatin on BMP-induced luciferase activity was determined. We observed that addition of myostatin potently blocked BMP7-induced I-BRE reporter activity (Fig. 4A). In sharp contrast, activation of I-BRE by BMP2 was not blocked by myostatin (Fig. 4B). Unlike myostatin, TGF-β decreased I-BRE-Lux activation by both BMP7 and BMP2 (Fig. 4C and D). These results reinforce the notion that myostatin specifically antagonizes BMP7 signaling without affecting BMP2. To verify that the effect of myostatin was not promoter specific, we examined the effect of myostatin on BMP7-dependent activation of another reporter, Msx2-Lux (Fig. 4E), which is composed of a 3-kb promoter fragment of the BMP-regulated homeobox gene Msx2 (51). As with I-BRE-Lux, myostatin and TGF-β abrogated BMP7-dependent activation of Msx2-Lux in C3H 10T1/2 cells. Similar results were obtained using the Xenopus Vent2 promoter (data not shown).
FIG. 4.
Myostatin antagonizes BMP7 but not BMP2 signaling. (A to E) C3H 10T1/2 cells were transiently transfected with the I-BRE-Lux reporter (A to D) or Msx2-Lux (E), and luciferase activity in cells treated with 0.75, 1, or 1.5 nM BMP7 (A, C, and E) or BMP2 (B and D), with or without myostatin (1, 5, or 10 nM) or TGF-β (25, 50, or 100 pM) was determined. (F) Myostatin but not TGF-β blocks BMP7-induced phosphorylation of endogenous Smad1. C3H 10T1/2 cells were incubated for 1 h with 1 nM BMP7 or BMP2 in the presence of 10 nM myostatin or 100 pM TGF-β as indicated. Total cell lysates were immunoblotted with anti-phosphospecific Smad1 or anti-Smad1 antibodies. (G and H) Myostatin does not inhibit signaling by a constitutively activated ALK2 receptor. C3H 10T1/2 cells were transiently transfected with the I-BRE-Lux reporter, and increasing amounts (0.001, 0.002, and 0.01 μg/well) of the activated type I receptor ALK2QD (G) or were treated with 1 nM BMP7 ligand, and myostatin (10, 15, or 20 nM) or TGF-β (50, 75, or 100 pM) (H), and the luciferase activity was measured.
Activation of BMP signaling results in phosphorylation of Smad1; therefore, we next determined whether myostatin altered this process. As expected, treatment of C3H 10T1/2 cells with BMP7 or BMP2 resulted in phosphorylation of endogenous Smad1 (Fig. 4F). Interestingly, coincubation of cells with myostatin blocked BMP7- but not BMP2-induced Smad1 phosphorylation. This result is consistent with our observation that myostatin can block BMP7- but not BMP2-induced differentiation and luciferase reporter activity (Fig. 1 and 4). In contrast, TGF-β treatment had little effect on BMP2- or BMP7-induced Smad1 phosphorylation. Similar results were obtained with 3T3-L1 and C2C12 cells (data not shown).
Myostatin blocks BMP7 effects upstream of Smads.
R-Smads are directly phosphorylated by the type I receptor kinase; therefore, we next examined whether myostatin blocks Smad1 phosphorylation upstream or downstream of the type I receptor. For this we used a constitutively active version of the BMP7 type I receptor ALK2 (ALK2 QD), which signals in the absence of ligand and type II receptor. C3H 10T1/2 cells were transfected with the I-BRE-Lux reporter together with the activated ALK2 receptor, and the effect of myostatin and TGF-β on luciferase activity were examined. As expected, cotransfection of increasing amounts of the activated ALK2 receptor induced luciferase reporter activity in a dose-dependent manner (Fig. 4G). Treatment of cells with myostatin had a modest effect on ALK2 QD-mediated activation, whereas in parallel experiments, similar doses of ligand potently blocked BMP7-induced activation of the I-BRE-Lux reporter (Fig. 4G and H). Unlike myostatin, TGF-β potently decreased both ALK2 QD- and BMP7-induced transcriptional activation of the I-BRE-Lux reporter (Fig. 4G and H). These data, together with the Smad1 phosphorylation findings, suggest that myostatin blocks BMP7 activity upstream of type I receptor activation.
Myostatin blocks BMP7 but not BMP2 receptor binding.
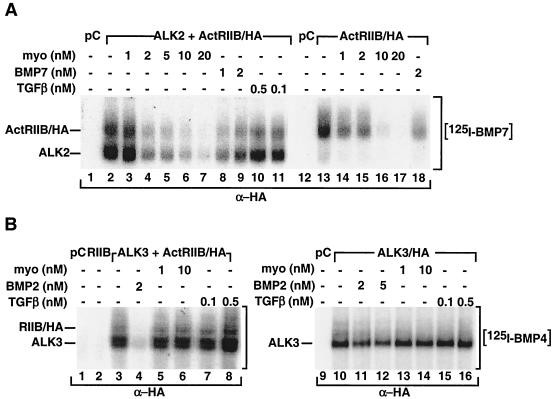
The finding that myostatin antagonizes BMP7 activity upstream of the type I receptor led us to postulate that myostatin might block the binding of BMP7 to its receptors. BMP7 can signal through either ALK2 or ALK6, but since undifferentiated C3H 10T1/2 cells do not express ALK6 (data not shown), we used ALK2 together with the type II partner ActRIIB for our studies. COS-1 cells transfected with these BMP7 receptors were affinity labeled with [125I]BMP7, and the effect of myostatin on [125I]BMP7 binding was examined. Consistent with previous work, BMP7 bound to the type II receptor, ActRIIB, but not the type I receptor, ALK2, when these receptors were expressed alone (Fig. 5A, lane 13, and data not shown). However, when the two receptors were coexpressed, efficient binding of BMP7 to ActRIIB and ALK2 was observed (lane 2). This binding was decreased in the presence of excess unlabeled BMP7 (lanes 8 and 9) as well as activin (1 nM) (data not shown). Furthermore, binding was also inhibited by increasing concentrations of myostatin (lanes 3 to 7) but not by excess unlabeled TGF-β (lanes 10 and 11). Myostatin was also effective in preventing BMP7 receptor binding to ActRIIB when it was expressed alone (lanes 14 to 17). Myostatin doses that were effective in competing for receptor binding were similar to those that resulted in antagonism of BMP7-induced adipogenic differentiation (Fig. 1). Together, these results indicate that myostatin competes with BMP7 for binding to ActRIIB and inhibits the formation of a BMP7-ActRIIB-ALK2 heteromeric receptor complex.
FIG. 5.
Myostatin inhibits BMP7 signaling by competing for receptor binding. (A and B) Myostatin blocks BMP7 but not BMP4 receptor binding. COS-1 cells were transiently transfected with various combinations of pCMV5 (pC), ActRIIB/HA (RIIB), and untagged ALK2 (A2) (A) or HA-tagged ALK3 (B) as indicated. The cells were incubated with 2 nM [125I]BMP7 or [125I]BMP4, and various concentrations of unlabeled myostatin, TGF-β, BMP7, or BMP2, as indicated. Cell lysates were subjected to anti-HA immunoprecipitation followed by SDS-PAGE, and bound ligand was visualized by autoradiography.
Since myostatin is ineffective in blocking BMP2 signaling, we next examined the effect of myostatin on BMP2/4 binding to its receptors by affinity labeling with [125I]BMP4. BMP2 and BMP4 primarily signal through ALK3 or ALK6, but since C3H 10T1/2 cells lack ALK6, we focused our studies on ALK3 along with the type II receptor ActRIIB. As expected, when the receptors were individually expressed, BMP4 interacted with ALK3 (Fig. 5B, lane 10) but not with ActRIIB (lane 2), whereas binding to both receptors could be detected when ALK3 and ActRIIB were coexpressed (lane 3). While BMP2 competed efficiently for binding to the receptor complex (lane 4), neither myostatin (lanes 5 and 6) nor TGF-β (lanes 7 and 8) prevented the association of [125I]BMP4 with the heteromeric receptor complex. Furthermore, while BMP2 could prevent the binding of [125I]BMP4 to ALK3 expressed alone (lanes 11 and 12), myostatin and TGF-β (lanes 13 to 16) did not. These results demonstrate that myostatin can inhibit BMP7 but not BMP2 receptor binding. Together, our findings indicate that myostatin potently antagonizes BMP7-induced signaling and differentiation by preventing BMP7 receptor engagement whereas it does not prevent BMP2 receptor activation. In contrast, TGF-β had no effect on the binding of either BMP2 or BMP7 to its receptor.
ActRIIB is the relevant BMP7 type II receptor in C3H 10T1/2 cells.
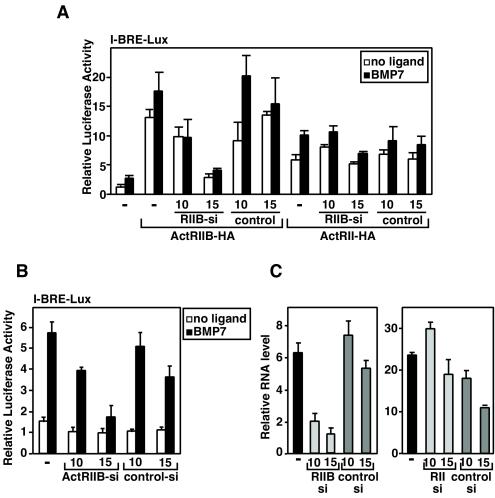
Our biochemical analysis demonstrates that myostatin can compete with BMP7 for binding to the type II receptor, ActRIIB. To determine whether this mechanism is likely to mediate the effect of myostatin on BMP7 signaling in C3H 10T1/2 cells, we used RNA interference technology to silence ActRIIB expression. To confirm that specific inhibition of ActRIIB-mediated BMP7 signaling was achieved, we first tested the effect of transfecting ActRIIB siRNA into C3H 10T1/2 cells together with either ActRII or ActRIIB receptor constructs. As is typically observed, overexpression of ActRIIB or ActRII increased both basal and BMP7-dependent activation of the I-BRE-Lux reporter (Fig. 6A). The ActRIIB-specific siRNA blocked signaling by transfected ActRIIB almost to control levels. In contrast, signaling by transfected ActRII was only minimally altered at the highest dose used. Cotransfection of an irrelevant siRNA did not affect either ActRIIB- or ActRII-mediated signaling (Fig. 6A). Thus, the siRNA specifically targets the ActRIIB receptor but not the ActRII receptor. We next examined the effect of ActRIIB-specific siRNA on BMP7-dependent activation of I-BRE-Lux through endogenous receptors in C3H 10T1/2 cells. The ActRIIB-specific siRNA potently decreased BMP7-induced activation of reporter activity, whereas an irrelevant siRNA control did not (Fig. 6B). Parallel analysis of mRNA levels by Q-PCR confirmed that ActRIIB but not ActRII transcript levels were decreased relative to control levels while the irrelevant control siRNA had minimal effects (Fig. 6C). Together, these results indicate that ActRIIB is primarily responsible for mediating BMP7-mediated signaling in C3H 10T1/2 cells.
FIG. 6.
RNA interference of ActRIIB abolishes BMP7 signaling in C3H 10T1/2 cells. C3H 10T1/2 cells were transiently transfected with the I-BRE-Lux reporter (A and B) without (B and C) or with (A) ActRII or ActRIIB, as well as various doses (0.8 or 1 μg/well) of ActRIIB-specific siRNA or an irrelevant control/scrambled siRNA (A to C). Luciferase activity of cells treated with BMP7 (1 nM) was assayed (A and B), and changes in ActRIIB and ActRII transcripts were measured by Q-PCR (C). In panel A, ActRIIB siRNA specifically reduces ActRIIB-mediated BMP7 signaling in C3H 10T1/2 cells without affecting ActRII-mediated signaling.
DISCUSSION
TGF-β superfamily members play an important role in the determination and differentiation of mesenchymal progenitors along the adipogenic, osteogenic, and chondrogenic pathways (12, 17, 18, 34, 58). While the signaling pathway for BMPs, TGF-βs, and activins has been extensively studied, little is known of the molecular mechanism of myostatin function. Since a physiological role for myostatin in both adipogenesis and myogenesis has been established (36, 38, 64), we focused on elucidating the myostatin signal transduction pathway. We demonstrate that myostatin signals through a TGF-β/activin/nodal-like pathway by binding to and activating a receptor complex composed of the type II receptor ActRIIB together with a type I receptor partner of either ALK4 or ALK5 (Fig. 7). The activated receptor phosphorylates Smad2 and Smad3, which then propagate the intracellular signal that ultimately leads to activation of TGF-β-responsive promoters. Although ligand-receptor promiscuity has been previously reported for activins, BMPs, and nodals, it is generally thought that TGF-β is the only ligand for TβRII and ALK5. Thus, our work provides the first demonstration that the TGF-β type I receptor ALK5 can also display promiscuous behavior. This observation has important implications for the identification of biologically relevant ligands in studies where ALK5 activity is disrupted, particularly in contexts in which TGF-β and myostatin are coexpressed.
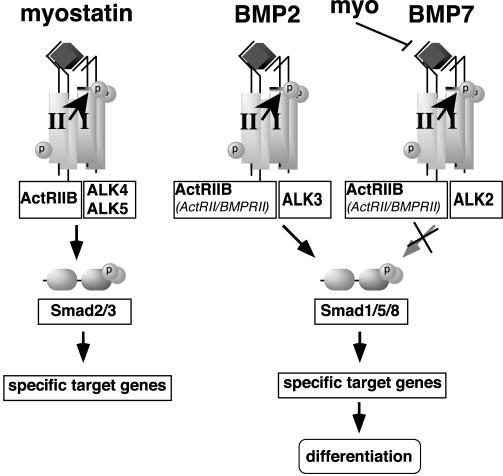
FIG. 7.
Model of extracellular and intracellular antagonism of BMP signaling by myostatin and TGF-β. Myostatin signals through ActRIIB and either ALK4 or ALK5 to activate a TGF-β-like signaling pathway. Myostatin potently antagonizes BMP7 but not BMP2 by competing for BMP7 binding to the ActRIIB type II receptor.
BMP activity is regulated extracellularly by secreted proteins such as chordin, noggin, gremlin, cereberus, DAN, and twisted gastrulation (6). Here we demonstrate that myostatin can also act extracellularly to antagonize BMP7 activity by preventing BMP7-receptor binding (Fig. 7). However, myostatin does not affect BMP2-receptor engagement. Interestingly, binding of BMP ligands to cell surface receptors occurs through two distinct mechanisms. BMP7, like TGF-β and activin, first binds to the type II receptor and then recruits a type I receptor to form a type II/I heteromeric receptor complex. In contrast, BMP2 and BMP4 bind to the type I receptor alone, but in the presence of the type II receptor, a high-affinity BMP2/BMP4 heteromeric receptor complex is formed. In our study, we showed that myostatin can block BMP7 but not BMP2 signaling. The distinct mechanisms of BMP binding and formation of heteromeric receptor complexes most probably explain the differential effects of myostatin on BMP7 versus BMP2 and BMP4 receptor binding. While myostatin can bind to the type II receptor ActRIIB, it cannot bind to type I receptors when they are expressed alone. Since the first step in the formation of a BMP7 heteromeric receptor complex is binding of BMP7 to the type II receptor, myostatin binding to this common receptor is an effective blocker of BMP7-receptor complex formation. In the case of BMP2 and BMP4, the binding of BMP2 to the type I receptor appeared to successfully compete with free myostatin for binding to the common type II receptor. Therefore, it seems likely that the different modes of BMP ligand binding or recruitment of heteromeric receptor complexes determines how effective myostatin might be in blocking their activities. Of note, activin and BMP7 have been reported to antagonize each other's activities by competing for the common type II receptor ActRII, although in this study, the ability of activin to compete with BMP2 binding was not examined (46). In contrast, inhibin can block BMP binding to both ActRII and ActRIIB (56a).
The ability of TGF-β superfamily members including BMP2, BMP4, BMP7, and TGF-β to regulate mesenchymal cell differentiation is well documented (17, 58). For instance, BMP2 and BMP7 can promote adipogenesis, osteogenesis, and chondrogenesis whereas TGF-β has most often been shown to block these differentiation processes. Since TGF-β utilizes receptors distinct from those required by BMPs and did not block Smad1 phosphorylation in our assays, it is likely that TGF-β antagonizes differentiation through a distinct intracellular mechanism. As myostatin and TGF-β utilize similar intracellular signaling cascades, it would seem that both ligands should be able to utilize this common intracellular mechanism to inhibit differentiation, and perhaps myostatin does so. However, the ability of myostatin to block BMP7 extracellularly provides for an important distinction. Cells exposed to both BMP7 and TGF-β would activate both Smad1 and Smad2, which are opposing pathways, and the final determination of cell fate may depend on the relative balance between the two signals. In contrast, by blocking Smad1 phosphorylation as well as activating the Smad2 pathway, myostatin provides for a pure TGF-β-like signal and may thereby have a more potent or perhaps distinct effect on differentiation. Presumably, this distinction would be lost in BMP-independent differentiation processes. Interestingly, there are many examples in the literature that demonstrate that TGF-β effects on differentiation, presumably through this intracellular pathway, can vary significantly depending on external factors such as cell type, density of plating, length of treatment, and medium composition (for example see references 14, 20, 21, and 63). The mechanism of this intracellular block remains unclear, although the possibilities include competition for limiting quantities of the common Smad, Smad4 (55), Smad-dependent regulation of specific transcriptional events (2, 29), and TGF-β-mediated activation of distinct Smad pathways through distinct ALKs (44). The relative contributions of myostatin-mediated extracellular versus intracellular competition in vivo remain to be determined; however, a more detailed understanding of the intracellular pathway of antagonism is required before this can be examined.
In addition to ActRIIB, both BMP2 and BMP7 signal through the type II receptors ActRII and BMPRII. Similarly, activin can bind equivalently to ActRII and ActRIIB. In contrast, myostatin displays a marked preference for ActRIIB over ActRII (27; also see above), which is consistent with the idea that there are distinct functions for these two related receptors. In the context of BMP receptor antagonism, binding of myostatin primarily to ActRIIB suggests that myostatin would be an ineffective extracellular inhibitor of BMP7 signaling through the other type II receptors. While ActRIIB contributes significantly to BMP7-induced responses in our model system (C3H 10T1/2 cells), other lineages may preferentially signal through ActRII or BMPRII. Therefore, a receptor-dependent differential ability of myostatin to block BMP7 activity may be particularly important in certain biological contexts, where changes in expression patterns of these type II receptors may determine whether BMP7 signals and associated differentiation events are negatively regulated by myostatin. Consistent with this possibility, ActRII and ActRIIB display both common and distinct expression patterns in the developing chicken limb (39, 42). Although extremely speculative, it may be that BMP-dependent patterning of bone and cartilaginous elements within the limb bud may be refined by myostatin, which is expressed in the myotome layer of adjacent somites that migrate into the limb to form the musculature (36). By differentially regulating BMPs and by functioning only in the context of appropriate receptor combinations, myostatin may provide for exquisite control of complex differentiation processes. Although BMPs have been implicated in the control of muscle growth and positioning during embryonic development, their importance in adult muscle or fat homeostasis is not currently known. However, it is intriguing to speculate that muscle mass or fat accumulation in adults might also be controlled by a balance between myostatin and BMP. If so, it will be important to consider how systemic administration of myostatin might alter BMP-dependent events in adults.
Acknowledgments
We thank K. Cho (Xvent2), S. Ghosh (Msx2-Lux), and B. Vogelstein (SBE4-Lux) for plasmids.
This work was supported by grants to L.A. and J.L.W. from the Canadian Institute for Health Research (CIHR). H.B. is the recipient of a studentship from NSERC and the Heart and Stroke Foundation of Canada. L.A. and J.L.W. are CIHR Investigators.
REFERENCES
- 1.Aherns, M., T. Ankenbauer, D. Schroder, A. Hollnagel, H. Mayer, and G. Gross. 1993. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H 10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 12:871-880. [DOI] [PubMed] [Google Scholar]
- 2.Alliston, T., L. Choy, P. Ducy, G. Karsenty, and R. Derynck. 2001. TGF-β-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 20:2254-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahina, I., T. K. Sampath, and P. V. Hauschka. 1996. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp. Cell Res. 222:38-47. [DOI] [PubMed] [Google Scholar]
- 4.Attisano, L., J. Cárcamo, F. Ventura, F. M. B. Weis, J. Massagué, and J. L. Wrana. 1993. Identification of human activin and TGF-β type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75:671-680. [DOI] [PubMed] [Google Scholar]
- 5.Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-β superfamily. Science 296:1646-1647. [DOI] [PubMed] [Google Scholar]
- 6.Balemans, W., and W. Van Hul. 2002. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev. Biol. 250:231-250. [PubMed] [Google Scholar]
- 7.Benchabane, H., and J. L. Wrana. 2003. GATA and Smad1-dependent enhancers in the Smad7 gene differentially interpret BMP gradients. Mol. Cell. Biol. 23:6646-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanovich, S., T. O. Krag, E. R. Barton, L. D. Morris, L. A. Whittemore, R. S. Ahima, and T. S. Khurana. 2002. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420:418-421. [DOI] [PubMed] [Google Scholar]
- 9.Brummel, T., V. Twombly, G. Marques, J. L. Wrana, S. Newfeld, L. Attisano, J. Massagué, M. B. O'Connor, and W. M. Gelbart. 1994. Characterization and relationship of dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell 78:251-261. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X., M. J. Rubock, and M. Whitman. 1996. A transcriptional partner for MAD proteins in TGF-β signalling. Nature 383:691-696. [DOI] [PubMed] [Google Scholar]
- 11.Colavita, A., S. Krishna, H. Zheng, R. W. Padgett, and J. G. Culotti. 1998. Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science 281:706-709. [DOI] [PubMed] [Google Scholar]
- 12.Derynck, R., and L. Choy. 1998. Transforming growth factor-β and its receptors, p. 593-636. In A. W. Thomson (ed.), The cytokine handbook, 3rd ed. Academic Press, Inc., Orlando, Fla.
- 13.Fajas, L., J. C. Fruchart, and J. Auwerx. 1998. Transcriptional control of adipogenesis. Curr. Opin. Cell Biol. 10:165-173. [DOI] [PubMed] [Google Scholar]
- 14.Filvaroff, E. H., R. Ebner, and R. Derynck. 1994. Inhibition of myogenic differentiation in myoblasts expressing a truncated type II TGF-beta receptor. Development 120:1085-1095. [DOI] [PubMed] [Google Scholar]
- 15.Hansen, J. B., R. K. Petersen, B. M. Larsen, J. Bartkova, J. Alsner, and K. Kristiansen. 1999. Activation of peroxisome proliferator-activated receptor gamma bypasses the function of the retinoblastoma protein in adipocyte differentiation. J. Biol. Chem. 274:2386-2393. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi, H., S. Abdollah, Y. Qiu, J. Cai, Y.-Y. Xu, B. W. Grinnell, M. A. Richardson, J. N. Topper, M. A. Gimbrone, Jr., J. L. Wrana, and D. Falb. 1997. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89:1165-1173. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman, A., and G. Gross. 2001. BMP signaling pathways in cartilage and bone formation. Crit. Rev. Eukaryot. Gene Expr. 11:23-45. [PubMed] [Google Scholar]
- 18.Hogan, B. L. 1996. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 7:856. [DOI] [PubMed] [Google Scholar]
- 19.Hoodless, P. A., T. Haerry, S. Abdollah, M. Stapleton, M. B. O'Connor, L. Attisano, and J. L. Wrana. 1996. MADR1, a MAD-related protein that functions in BMP2 signalling pathways. Cell 85:489-500. [DOI] [PubMed] [Google Scholar]
- 20.Ignotz, R. A., and J. Massagué. 1985. Type β transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA 82:8530-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katagiri, T., A. Yamaguchi, M. Komaki, E. Abe, N. Takahashi, T. Ikeda, V. Rosen, J. M. Wozney, A. Fujisawa-Sehara, and T. Suda. 1994. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127:1755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, K. H., K. Lee, Y. S. Moon, and H. S. Sul. 2001. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J. Biol. Chem. 276:11252-11256. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch, T., J. Nickel, and W. Sebald. 2000. BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II. EMBO J. 19:3314-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenig, B. B., J. S. Cook, D. H. Wolsing, J. Ting, J. P. Tiesman, P. E. Correa, C. A. Olson, A. L. Pecquet, F. Ventura, R. A. Grant, G.-X. Chen, J. L. Wrana, J. Massagué, and J. S. Rosenbaum. 1994. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol. 14:5961-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapsys, N. M., A. D. Kriketos, M. Lim-Fraser, A. M. Poynten, A. Lowy, S. M. Furler, D. J. Chisholm, and G. J. Cooney. 2000. Expression of genes involved in lipid-metabolism correlate with peroxisome proliferator-activated receptor gamma expression in human skeletal muscle. J. Clin. Endocrinol. Metab. 85:4293-4297. [DOI] [PubMed] [Google Scholar]
- 26.Lee, S.-J., and A. C. McPherron. 1999. Myostatin and the control of skeletal muscle mass. Curr. Op. Genet. Dev. 9:604-607. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S.-J., and A. C. McPherron. 2001. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 98:9306-9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, J., H. B. Arnold, M. A. Della-Fera, M. J. Azain, D. L. Hartzell, and C. A. Baile. 2002. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem. Biophys. Res. Commun. 291:701-706. [DOI] [PubMed] [Google Scholar]
- 29.Liu, D., B. L. Black, and R. Derynck. 2001. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 15:2950-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, F., F. Ventura, J. Doody, and J. Massagué. 1995. Human type II receptor for bone morphogenetic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 15:3479-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo, P. C. H., and M. Frasch. 1999. Sequence and expression of myoglianin, a novel Drosophila gene of the TGF-β superfamily. Mech. Dev. 86:171-175. [DOI] [PubMed] [Google Scholar]
- 32.Macìas-Silva, M., P. A. Hoodless, S. J. Tang, M. Buchwald, and J. L. Wrana. 1998. Specific activation of Smad1 signalling pathways by the BMP7 type I receptor, ALK 2. J. Biol. Chem. 273:25628-25636. [DOI] [PubMed] [Google Scholar]
- 33.Massagué, J. 1998. TGF-β signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 34.Massagué, J. 1990. The transforming growth factor-β family. Annu. Rev. Cell Biol. 6:597-641. [DOI] [PubMed] [Google Scholar]
- 35.McPherron, A. C., A. M. Lawler, and S.-J. Lee. 1999. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 22:260-264. [DOI] [PubMed] [Google Scholar]
- 36.McPherron, A. C., A. M. Lawler, and S. J. Lee. 1997. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83-90. [DOI] [PubMed] [Google Scholar]
- 37.McPherron, A. C., and S.-J. Lee. 1997. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 94:12457-12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPherron, A. C., and S. J. Lee. 2002. Suppresion of body fat accumulation in myostatin-deficient mice. J. Clin. Investig. 109:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merino, R., D. Macias, Y. Gañan, J. Rodriguez-Leon, A. N. Economides, C. Rodriguez-Esteban, J. C. Izpisua-Belmonte, and J. M. Hurle. 1999. Control of digit formation by activin signalling. Development 126:2161-2170. [DOI] [PubMed] [Google Scholar]
- 40.Moustakas, A., S. Souchelnytskyi, and C.-H. Heldin. 2001. Smad regulation in TGF-β signal transduction. J. Cell Sci. 114:4359-4369. [DOI] [PubMed] [Google Scholar]
- 41.Nohno, T., T. Ishikawa, T. Saito, K. Hosokawa, S. Noji, D. H. Wolsing, and J. S. Rosenbaum. 1995. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J. Biol. Chem. 270:22522-22526. [DOI] [PubMed] [Google Scholar]
- 42.Nohno, T., S. Noji, E. Koyama, F. Myokai, H. Ohuchi, K. Nishikawa, S. Sumitomo, S. Taniguchi, and T. Saito. 1993. Expression patterns of the activin receptor IIA and IIB genes during chick limb development. Prog. Clin. Biol. Res. 383B:705-714. [PubMed] [Google Scholar]
- 43.Novikoff, A. B., P. M. Novikoff, O. M. Rosen, and C. S. Rubin. 1980. Organelle relationships in cultured 3T3-L1 preadipocytes. J. Cell Biol. 87:180-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh, S. P., T. Seki, K. A. Goss, T. Imamura, Y. Yi, P. K. Donahoe, L. Li, K. Miyazono, P. ten Dijke, S. Kim, and E. Li. 2000. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 97:2626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penton, A., Y. Chen, K. Staehling-Hampton, J. L. Wrana, L. Attisano, J. Szidonya, A. Cassill, J. Massagué, and F. M. Hoffmann. 1994. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell 78:239-250. [DOI] [PubMed] [Google Scholar]
- 46.Piek, E., M. Afrakhte, K. Sampath, E. J. Van Zoelen, C.-H. Heldin, and P. ten Dijke. 1999. Functional antagonism between activin and osteogenic protein-1 in human embryonal carcinoma cells. J. Cell. Physiol. 180:141-149. [DOI] [PubMed] [Google Scholar]
- 47.Reissmann, E., H. Jörnvall, A. Blokzijl, O. Andersson, C. Chang, G. Minchiotti, M. G. Persico, C. F. Ibánez, and A. H. Brivanlou. 2001. The orphan receptor ALK7 and the activin receptor ALK4 mediate signaling by nodal proteins during vertebrate development. Genes Dev. 15:2010-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen, E. D., and B. M. Spiegelman. 2000. Molecular recognition of adipogenesis. Annu. Rev. Cell Dev. Biol. 16:145-171. [DOI] [PubMed] [Google Scholar]
- 49.Rosenzweig, B. L., T. Imamura, T. Okadome, G. N. Cox, H. Yamashita, P. ten Dijke, C. H. Heldin, and K. Miyazono. 1995. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 92:7632-7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma, M., R. Kambadur, K. G. Matthews, W. G. Somers, G. P. Devlin, J. V. Conaglen, P. J. Fowke, and J. J. Bass. 1999. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell. Physiol. 180:1-9. [DOI] [PubMed] [Google Scholar]
- 51.Sirard, C., S. Kim, C. Mirtsos, P. Tadich, P. A. Hoodless, A. Itie, R. Maxson, J. L. Wrana, and T. W. Mak. 2000. Targeted disruption in murine cells reveals variable requirement for Smad4 in transforming growth factor β-related signaling. J. Biol. Chem. 275:2063-2070. [DOI] [PubMed] [Google Scholar]
- 52.Steuerwald, N., J. Cohen, R. J. Herrera, and C. A. Brenner. 2000. Quantification of mRNA in single oocytes and embryos by real-time rapid cycle fluorescence monitored RT-PCR. Mol. Hum. Reprod. 6:448-453. [DOI] [PubMed] [Google Scholar]
- 53.ten Dijke, P., H. Yamashita, T. K. Sampath, A. H. Reddi, M. Estevez, D. L. Riddle, H. Ichijo, C.-H. Heldin, and K. Miyazono. 1994. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 269:16985-16988. [PubMed] [Google Scholar]
- 54.Thies, R. S., T. Chen, M. V. Davies, K. N. Tomkinson, A. A. Pearson, Q. A. Shakey, and N. M. Wolfman. 2001. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors 19:251-259. [DOI] [PubMed] [Google Scholar]
- 55.von Bubnoff, A., and K. W. Cho. 2001. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev. Biol. 239:1-14. [DOI] [PubMed] [Google Scholar]
- 56.Wang, E. A., D. I. Israel, S. Kelly, and D. P. Luxenberg. 1993. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9:57-71. [DOI] [PubMed] [Google Scholar]
- 56a.Wiater, E., and W. Vale. 2003. Inhibin is an antagonist of bone morphogenetic protein signaling. J. Biol. Chem. 278:7934-7941. [DOI] [PubMed] [Google Scholar]
- 57.Wrana, J. L., L. Attisano, J. Carcamo, A. Zentella, J. Doody, M. Laiho, X.-F. Wang, and J. Massagué. 1992. TGFβ signals through a heteromeric protein kinase receptor complex. Cell 71:1003-1014. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi, A. 1995. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-beta superfamily. Semin. Cell. Biol. 6:165-173. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita, H., P. ten Dijke, D. Huylebroeck, T. K. Sampath, M. Andries, J. C. Smith, C.-H. Heldin, and K. Miyazono. 1995. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 130:217-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, J., T. Ratovitski, J. P. Brady, M. B. Solomon, K. D. Wells, and R. J. Wall. 2001. Expression of myostatin pro domain results in muscular transgenic mice. Mol. Reprod. Dev. 60:351-361. [DOI] [PubMed] [Google Scholar]
- 61.Yeo, C.-Y., and M. Whitman. 2001. Nodal signals to Smads through cripto-dependent and cripto-independent mechanisms. Mol. Cell 7:949-957. [DOI] [PubMed] [Google Scholar]
- 62.Zawel, L., J. L. Dai, P. Buckhaults, S. Zhou, K. W. Kinzler, B. Vogelstein, and S. E. Kern. 1998. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell 1:611-617. [DOI] [PubMed] [Google Scholar]
- 63.Zentella, A., and J. Massagué. 1992. TGF-β induces myoblast differentiation in the presence of mitogens. Proc. Natl. Acad. Sci. USA 89:5176-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmers, T. A., M. V. Davies, L. G. Koniaris, P. Haynes, A. F. Esquela, K. N. Tomkinson, A. C. McPherron, N. M. Wolfman, and S.-J. Lee. 2002. Induction of cachexia in mice by systemically administered myostatin. Science 296:1486-1488. [DOI] [PubMed] [Google Scholar]