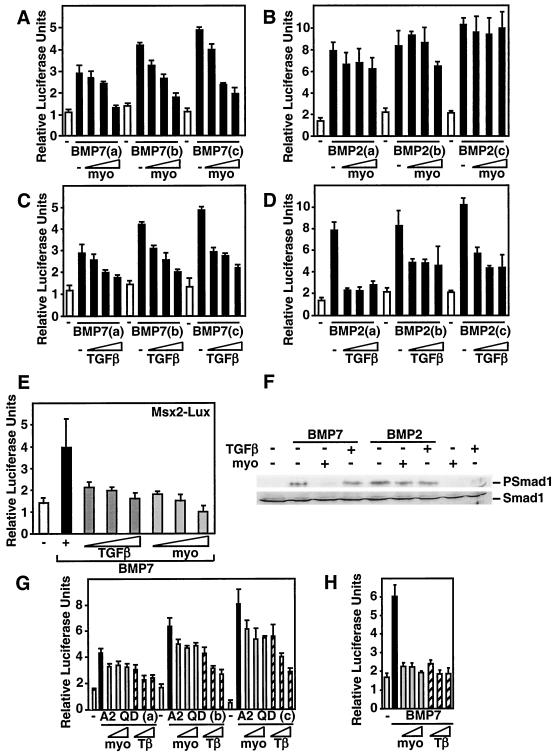
FIG. 4.
Myostatin antagonizes BMP7 but not BMP2 signaling. (A to E) C3H 10T1/2 cells were transiently transfected with the I-BRE-Lux reporter (A to D) or Msx2-Lux (E), and luciferase activity in cells treated with 0.75, 1, or 1.5 nM BMP7 (A, C, and E) or BMP2 (B and D), with or without myostatin (1, 5, or 10 nM) or TGF-β (25, 50, or 100 pM) was determined. (F) Myostatin but not TGF-β blocks BMP7-induced phosphorylation of endogenous Smad1. C3H 10T1/2 cells were incubated for 1 h with 1 nM BMP7 or BMP2 in the presence of 10 nM myostatin or 100 pM TGF-β as indicated. Total cell lysates were immunoblotted with anti-phosphospecific Smad1 or anti-Smad1 antibodies. (G and H) Myostatin does not inhibit signaling by a constitutively activated ALK2 receptor. C3H 10T1/2 cells were transiently transfected with the I-BRE-Lux reporter, and increasing amounts (0.001, 0.002, and 0.01 μg/well) of the activated type I receptor ALK2QD (G) or were treated with 1 nM BMP7 ligand, and myostatin (10, 15, or 20 nM) or TGF-β (50, 75, or 100 pM) (H), and the luciferase activity was measured.