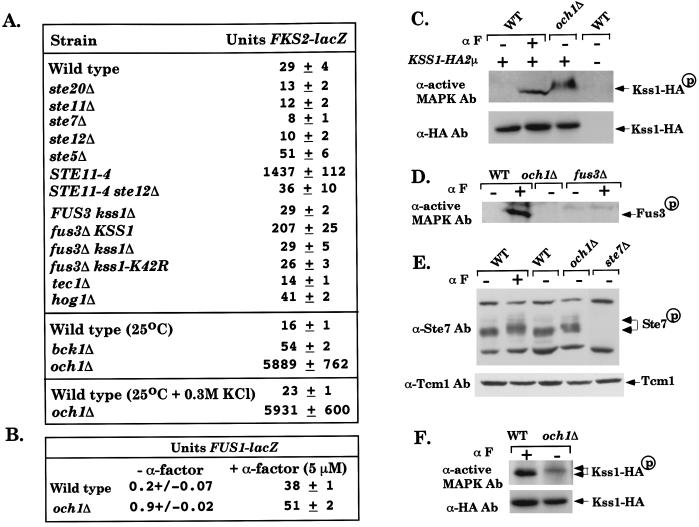
Figure 3.
The SVG pathway regulates FKS2 and is activated in an och1 mutant. (A) STE20, STE11, STE7, STE12, and TEC1 are required for FKS2 expression. Strains: PY1181 (WT), BLY409 (ste20Δ), BLY21 (ste11Δ), FP56 (ste7Δ), BLY380 (ste12Δ), BLY263 (ste5Δ), EY1298 (STE11–4 far1Δ), BLY426 (STE11–4 ste12Δ far1Δ), BLY405 (kss1Δ), BLY407 (fus3Δ), EY966 (fus3Δ kss1Δ), BLY535 (fus3Δ kss1Δ + pKSS1K42R-HIS3), BLY369 (tec1Δ), BLY457 (hog1Δ), C699-59 (bck1Δ), and BLY 341 (och1Δ). All strains contained FKS2-lacZ on a 2μ plasmid (pDM5) (33) and were grown in SC − uracil at 30°C or at 25°C, with or without 0.3 M KCl where indicated. β-Galactosidase activity (Miller units) was assayed in triplicate, and the average ± SD of three experiments is shown. (B) FUS1-lacZ expression is not induced in the och1Δ mutant. PY1181 (WT) and BLY470 (och1Δ) containing FUS1-lacZ on a CEN plasmid, induced for 120 min with α factor. (C) Kss1-HA3 is constitutively phosphorylated in the och1Δ mutant. Kss1-HA3 is on a 2μ plasmid (7). α F indicates α factor. The altered mobility of Kss1-HA3-P from the och1Δ strain is because of gel conditions (see F). (D) Fus3 is not phosphorylated in the och1Δ mutant. (E) Ste7 is hyperphosphorylated in an och1Δ mutant. Arrows indicate the portion of Ste7 that is shifted upward in lanes 2 and 4. (F) Mobility of phosphorylated Kss1-HA3 in OCH1 and och1Δ strains. Samples in C, D, and F are 50% saturated ammonium sulfate cuts of 2 mg of whole cell extract. Samples in E are 50 μg of whole cell extract.