Figure 3.
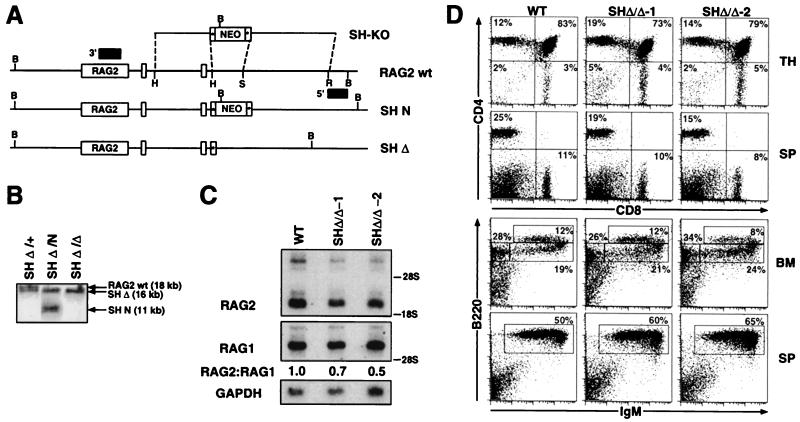
SHΔ/Δ chimeric mice exhibit normal B and T cell development and a minor reduction in thymic expression of RAG2. (A) A targeting vector (SH-KO) was designed to delete the endogenous StuI–HindIII (2-kb SH) region located 5′ of the RAG2 gene, employing sequences 5′ and 3′ of the StuI and HindIII sites to direct homologous recombination and a loxP-flanked PGK-neor gene. Also indicated are BamHI (B), EcoRI (R), StuI (S), and HindIII (H) restriction sites; loxP sites (starred boxes); and probes used for analysis of 5′ and 3′ homologous recombination (shaded boxes). The endogenous RAG2 allele (RAG2 wt) is depicted with the SH-KO vector and alleles in which the SH region has been replaced with either the PGK-neor gene (SH N) or a single loxP site (SH Δ), following Cre-mediated deletion. (B) Southern blot analysis with the 3′ probe on BamHI-digested DNA isolated from (i) ES cells in which the SH region had been targeted and replaced on one allele with a loxP site (SH Δ/+), (ii) SH Δ/+ ES cells targeted on the second allele with the PGK-neor gene (SH Δ/N), and (iii) SH Δ/N ES cells following Cre-mediated deletion of the PGK-neor gene (SH Δ/Δ). (C) Northern blot analysis of thymocyte RNA isolated from wild-type mice and SHΔ/Δ:RAG2−/− chimeric mice for the presence of RAG2 transcripts. Following hybridization with a RAG2 cDNA probe, the blot was stripped and rehybridized with a RAG1 cDNA probe to normalize levels of thymocyte RNA. The relative ratios of RAG2 to RAG1 hybridizing transcripts are shown. Hybridization with a GAPDH probe is included as an RNA loading control. (D) Thymus (TH), BM, and splenic (SP) cells from wild-type (WT) and SHΔ/Δ:RAG2−/− chimeric mice (generated from two independent SHΔ/Δ ES clones) were stained with (i) PE-anti-CD4 and CyC-anti-CD8, (ii) PE-anti-CD43 and CyC-anti-B220, or (iii) PE-anti-IgM and CyC-anti-B220 and analyzed by FACS. The percentages of total lymphocytes in various gated populations are shown from analysis of representative chimeras and WT mice.