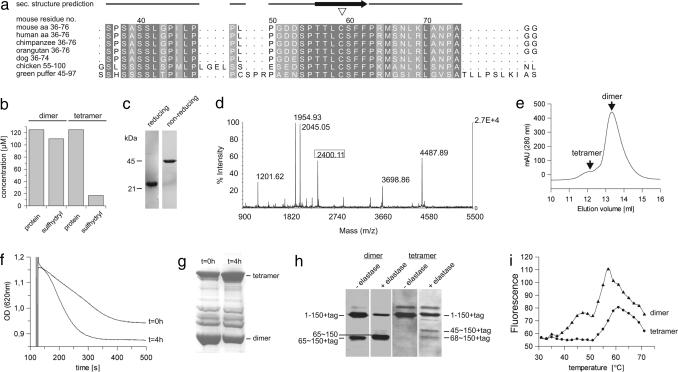
Fig. 2.
Disulfide bridging through the conserved residue Cys-59 and accompanied structural rearrangements in p66CH2-CB. (a) Alignment of p66CH2-CB from various organisms around the conserved Cys-59 residue (▿). (b) Detection of free sulfhydryl groups in the dimer but not in the tetramer form by using Ellman's reagent. (c) Tetrameric p66CH2-CB subjected to reducing and nonreducing SDS/PAGE. (d) MALDI-TOF-MS spectrum of the chymotryptic digest of the p66CH2-CB tetramer including the disulfide-linked peptide Leu-68–Cys-59–S–S–Cys-59′–Leu-68′ (framed). (e) SEC elution profile of the tetramer after treatment with 2.5 mM DTT. (f and g) Time-dependent increase in p66CH2-CB-induced rupture of mitochondria (f) and its correlation with the protein's oxidation level determined by nonreducing SDS/PAGE (g). Dimeric p66CH2-CB was analyzed (t = 0), subsequently incubated on ice for 4 h, and then tested again (t = 4 h). (h) Limited proteolysis of p66CH2-CH dimer and tetramer with elastase. Even before the addition of elastase, fragmentation of the dimer but not the tetramer was observed, caused by proteases of the expression host. (i) Thermal denaturation of p66CH2-CH dimer and tetramer.