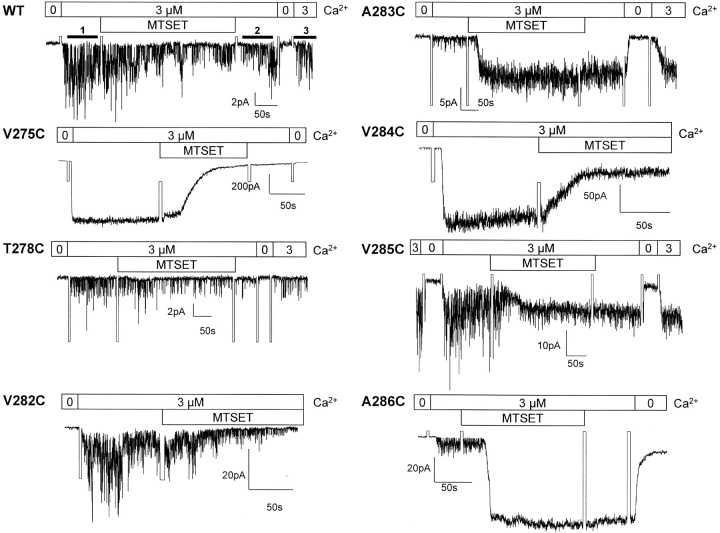
Figure 3.
Inside-out recordings illustrating the action of MTSET on IKCa mutants. Inside-out current records measured in symmetrical 200 mM K2SO4 + 3 μM internal Ca2+ conditions. The pipette potential was 60 mV throughout. The effect of the MTSET reagent on channel activity was estimated from the ratio <I>(test)/<I>(ctr) where <I>(ctr) is the mean current measured in 3 μM Ca2+ before drug application (labeled line 1 in WT) and <I>(test), the mean current obtained at the same Ca2+ concentration (labeled line 3 in WT) after the sequential washout of the drug with a Ca2+-containing (3 μM) (labeled line 2 in WT) and a Ca2+-free 200 mM K2SO4 solution. The drug was systematically applied for 5 min with a total washout period of 2 min. This procedure ensures that the observed effects of the MTS reagents are resulting from a covalent binding of the drug to the targeted cysteine, and not from nonspecific channel interactions with the open or closed channel. Strong inhibition (>75%) of channel activity was observed with V275C, T278C, and V282C after exposure to MTSET (5 mM) for 5 min, with a complete inhibition recorded with the V275C and V282C mutants. The V284C channel showed a maximal inhibition of 50% despite a steady-state current value reached after 45 s. Notably, MTSET caused an increase in inward currents when applied on the A283C, A286C, and to a lesser extent V285C mutants. The current increase remained Ca2+ and clotrimazole sensitive, ruling out nonspecific effects of MTSET. There were no significant variations in mean currents with A279C suggesting that this residue may not be MTSET accessible.