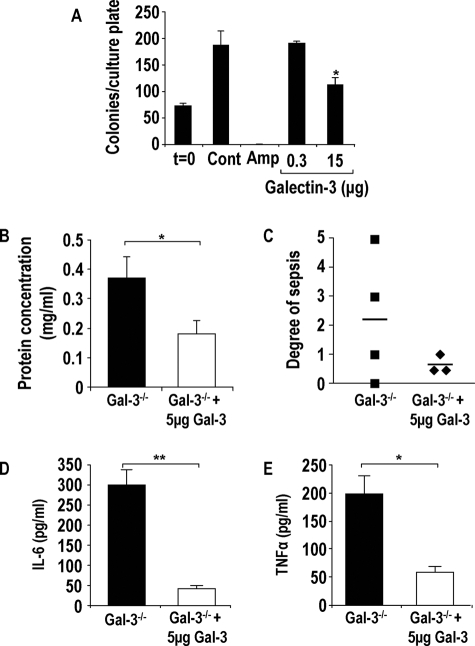
Figure 7.
Galectin-3 is bacteriostatic, and delivery of recombinant galectin-3 into the lungs of galectin-3−/− mice reduces severity of pneumonia. A: S. pneumoniae cultures were incubated with galectin-3 (0.3 and 15 μg) or ampicillin (20 μg/ml) for 2 hours, diluted 1:10, and plated onto blood agar plates. Colonies were counted the following day (n = 3) (*P < 0.05 compared to control). Galectin-3-deficient mice were intratracheally inoculated with 1 ×105 CFU S. pneumoniae in the presence or absence of 5 μg of recombinant mouse galectin-3 for 15 hours. B: Protein concentration was significantly higher in lavage fluid from galectin-3−/− (black bars) mice compared with galectin-3−/− mice treated with galectin-3 (white bars) (*P < 0.05 compared to galectin-3−/−). C: Blood from galectin-3−/− and WT mice was plated on blood agar plates, and the degree of bacterial presence per plate was scored according to the arbitrary scale 0 to 5 (0, no bacterial growth; 5, significant bacterial growth). Galectin-3−/− mice treated with galectin-3 demonstrated reduced sepsis compared to untreated galectin-3−/− mice. IL-6 (D) and TNF-α (E) concentration in BAL was determined by CBA and demonstrated to be lower in galectin-3-treated galectin-3−/− mice (**P < 0.01 compared to galectin-3−/− and *P < 0.05 compared to galectin-3−/−).