Figure 5.
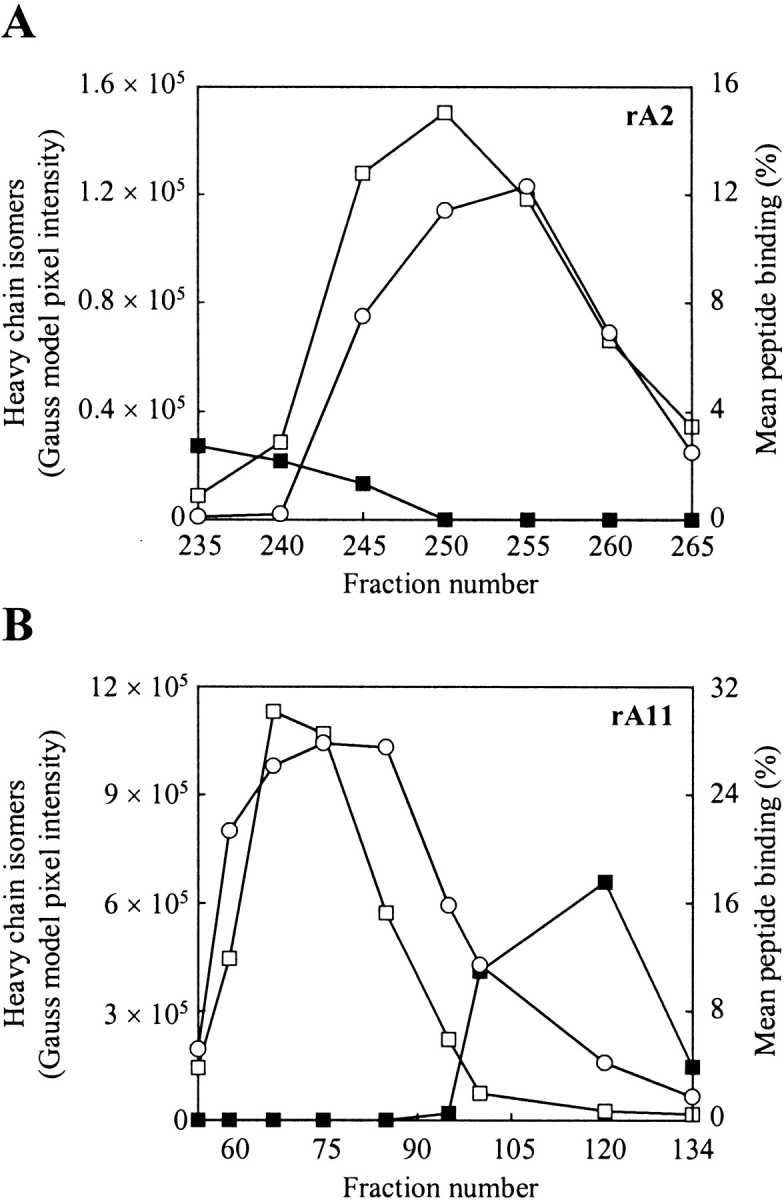
Refolding and peptide binding analysis of fractionated MHC-I heavy chain isomers. (A) Analysis of fractions collected during purification of rA2 on phenyl Sepharose High Performance (see Fig. 3 ▶). (B) Analysis of fractions collected during purification of rA11 on phenyl Sepharose High Performance (see Fig. 4 ▶). Folding was initiated by diluting aliquots (1 μL) from selected fractions 100-fold in 100 mM Tris-maleate (pH 6.6) buffer, containing human β2m (3 μM) and a radiolabeled peptide (15,000 cpm). The pixels intensities of heavy-chain isomers 1 and 2 were calculated from a densitometric analysis of SDS–polyacrylamide gels shown in Figure 3 and Figure 4 ▶ ▶, respectively. Fraction numbers are shown on the figure. Empty squares indicate isomer 1 protein tracing; solid squares, isomer 2 protein tracing; and circles, mean peptide binding. The standard deviation of duplicate peptide binding measurements was typically within 5%.
