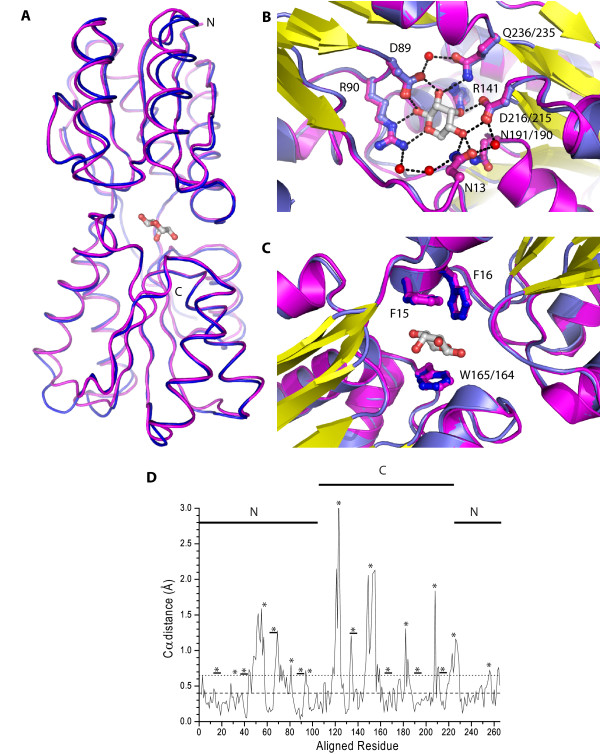
Figure 4.
Similarity between ecRBP and tteRBP. (A) Backbone atom alignment of tteRBP (blue) and ecRBP (magenta). Loops which have high RMSD are indicated (1/residues 55–61, 2/residues 117–126, 3/residues 149–156). (B) Close-up view of the polar binding pocket residues in tteRBP (blue) and ecRBP (magenta). Ribose is shown in gray. Critical residues involved in ribose binding are indicated (where the tteRBP and ecRBP numbering are different, the former is given first). (C) Close-up view of the non-polar binding pocket amino acids of tteRBP (blue) and ecRBP (magenta). (D) Structural differences in the Cα positions of the aligned models of ecRBP and tteRBP generated by LSQMAN [60]. Dashed and dotted lines indicate the RMSD of 235/271 and 270/271 of the Cα atoms respectively of the aligned structures. The N- and C- terminal residues are indicated with a solid line. Loops and turns are indicated (asterisk), or loops (underlined asterisk) in the binding pocket region.