Abstract
Extensive alterations in cellular organization are known to accompany the responses of sensitized T cells to target cells presenting an antigen of interest. Now, equally if not more dramatic changes are found to take place in cells presenting an antigen. With the help of a spinophilin-GFP fusion protein, Bloom et al. (Bloom, O., J.J. Unternaehrer, A. Jiang, J.-S. Shin, L. Delamarre, P. Allen, and I. Mellman. 2008. J. Cell Biol. 181:203–211) have captured a remarkable polarization of the cellular architecture of dendritic cells presenting an antigen to T cells.
An elegant study using z stack reconstructions and quantitative microscopy ushered in a new era in the cellular biology of the immune response nearly a decade ago, when Monks et al. (1998) convincingly documented the existence of a reproducible supramolecular organization segregating adhesion and antigen recognition functions in concentrically organized spatial structures focused on the contact zone between B cells and T cells. A substantial body of work was quickly erected upon the scaffold of this discovery, and the dynamic interface between the antigen-presenting cell and the responding cell soon became known as the immunological synapse (Grakoui et al., 1999).
But despite this passing homage to nearly a century of precedent studies in the central nervous system, relatively little attention was paid to the events that might be taking place in the antigen-presenting cell. Perhaps this reflected views that analogies between immune cells and neurons could not be pushed very far, given the general opportunism of evolution and the very great differences in roles that the participating cell types could be expected to play. In a striking paper presented in this issue (see p. 203), Bloom et al. (2008) have mined the similarities with the architecture of the nervous system to great effect, demonstrating that within the most potent of the specialized cells that present antigens to T cells, a highly dynamic structural reorganization can be observed, and that the morphological changes that attend antigen presentation are at least as spectacular as those observed in responding T cells.
Bloom et al. (2008) demonstrate this point by documenting the redistribution of an interesting adaptor protein known as spinophilin, found by Allen et al., (1997) and Satoh et al. (1998) in the dendritic spines of neurons in the central nervous system. Dendritic spines are dynamic structures that fluctuate in density, size, and morphology in response to environmental perturbations that can be associated with learning, hormonal changes, and development. Spinophilin is a relatively large protein that contains an F-actin–binding domain and a PDZ domain and can be found enriched in synaptic junctions as well as adherens junctions in epithelium. Spinophilin null mice have smaller hippocampi and increased density of striatal dendritic spines compared with their wild-type littermates (Feng et al., 2000). One particularly important client protein of spinophilin is protein phosphatase 1, which is directed by spinophilin to postsynaptic junctions, where it modulates AMPA and NMDA receptor sensitivity (Feng et al., 2000), and to axonal microtubules, where it regulates the phosphorylation state of doublecortin, ensuring a nonbranched axonal morphology (Bielas et al., 2007).
Of particular interest for its relevance to the present findings is the presence of the spinophilin PDZ domain. PDZ domains typically recognize short consensus ligand sequences at the C-terminus of proteins. The domains are often found in scaffold proteins that affiliate with the cytoskeleton, forming bridges between the latter and membrane-associated ligands, and a large number of proteins with such domains have been identified in neuronal synapses (Kim and Sheng, 2004). The spinophilin PDZ domain is unusual in recognizing two types of C-terminal sequence, so-called type I and type II sequences; this dual recognition, called type V, allows the protein to coordinate AMPA receptor GluR2/3 subunits as well as NMDA receptor NR1C′, NR2A/B, and NR2C/D subunits (Kelker et al., 2007). In T cells, scaffold proteins with PDZ domains have both a positive and negative involvement in organizing the T cell response and help establish the overall polarity of the cell (Ludford-Menting et al., 2005). The thematic connection between cytoskeleton and membrane appears to be preserved in spinophilin, which bears an internal domain coordinating the third intracellular loop of some G protein–coupled receptors (GPCRs) and also interacts with the N-terminal domain of R4 subgroup members of the RGS family of GAP proteins (Wang et al., 2007).
By a conspiracy of terminology, the most effective antigen-presenting cells in the immune system are known as dendritic cells for their extensively arborized structures. After the pioneering efforts of Steinman and Cohn (1974) brought their unusual potency to light, dendritic cells became of considerable interest to immunologists and were found to exhibit a rich biology that enables the organism to sensitively respond to changes in the environment that herald the presence of pathogens (Steinman, 2007). Bloom et al. (2008) vividly document here the participation of dendritic cell spinophilin in antigen presentation through a variety of microscopic studies that illustrate dramatic changes in spinophilin distribution accompanying the formation of the immunological synapse. Although the impact of the spinophilin null phenotype on the extent of activation in the examples presented in this study is not as spectacular as the visible manifestation of cytostructural reorganization, the impact on activation is well documented and clearly points to a function for spinophilin in the creation of a highly effective immune response. In some ways, this is reminiscent of but not perfectly congruent with the behavior of another protein borrowed from the central nervous system by dendritic cells, plexin-A1. Plexins constitute a family of cell surface proteins that are known to act as receptors for semaphorins. In dendritic cells, plexin-A1 appears to be retained in an intracellular, presumably vesicular, compartment, making its way to the cell surface after TNFα stimulation, where it clusters in a multifocal pattern localizing to the T cell synapse (Fig. 1 A; Eun et al., 2006). The mechanism that controls this behavior, like that of spinophilin, is not well understood.
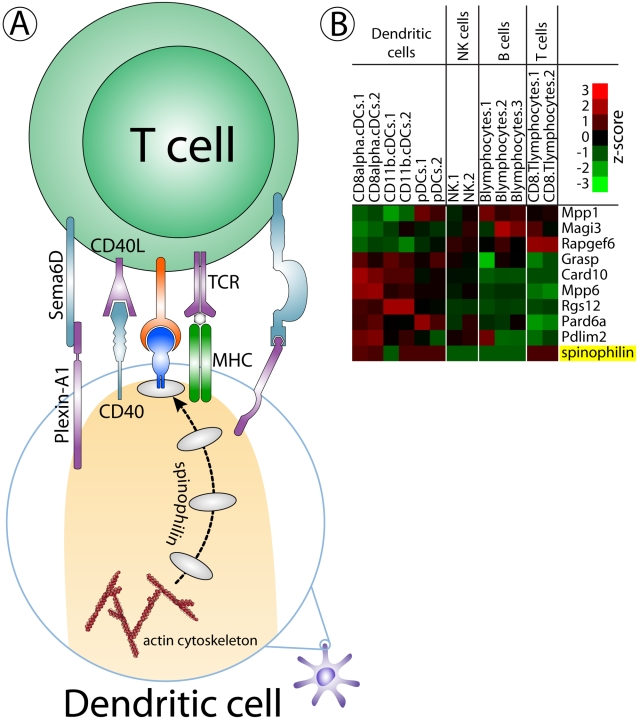
Figure 1.
Opportunities to mine the neuronal synaptic proteome for molecules of relevance to the immune synapse. (A) Schematic diagram of the interaction between a T cell and a dendritic cell that is presenting an antigen. When T cells encounter antigen-presenting cells, sequential recruitment of adhesion molecules and cytoplasmic signaling molecules contribute to the local assembly of the immune synapse. The adaptor protein spinophilin bears PDZ and actin-binding domains that allow it to undergo relocalization, presumably to engage or convey important client proteins, in the formation of the immune synapse. (B) Relative mRNA expression levels of proteins containing PDZ domains (candidate scaffold/adaptor proteins) that are differentially distributed between lymphocytes and dendritic cells.
If the complexity of the immune synapse approaches that of neuronal synapses, however, there will likely be many additional scaffold and adaptor proteins that will be found to subserve localizing and modulatory functions that are essential to the flow of information between the apposed cells. One potential approach to discovery in this area is the use of genome-wide profiling tools to uncover commonalities between gene expression patterns in neurons and antigen-presenting cells (Fig. 1 B).
Although it can be challenging to make appropriate assignments of importance or physiological involvement on the basis of initial measurements, at this point it is easy to defend the view that something unusual has been uncovered. And although the exact function of spinophilin in the immune response has yet to be elucidated, it is clear for the moment that the dendritic cell undergoes an internal structural reorganization at least as impressive, morphologically, as that found in the T cell upon antigen presentation, and that spinophilin is an excellent marker for cytostructural alterations of dendritic cells.
The spinophilin-GFP fusion protein deployed so effectively in this study may also prove to be a valuable tool for the examination of the dynamic responses of dendritic spines to local release of neurotransmitters in the central nervous system. Although spines do not exhibit the same plasticity of architecture that dendritic cells display, they can undergo dramatic volume increases, exceeding 100% in some cases, in a short period of time after intense excitation. Such changes are typically accompanied by a highly engaged signaling mediated by GPCRs and show long-term potentiation that exhibits coupling between adjacent spines, allowing neural circuitry to respond in a locally cohesive manner (Harvey and Svoboda, 2007). Much of the work on spinophilin to date has been mechanistic in focus and has elucidated connections that define the impact of the molecule on the primary actions of the GPCRs that mediate the postsynaptic response, but to date, the involvement of spinophilin in the structural dynamics of spines has not been strongly emphasized. The findings given here suggest that an exploration of this topic might well be fruitful.
Finally, for students of the antigen response, a host of questions have been raised. Among them: How does the dendritic cell sense the T cell? What other molecules are drawn into the presenting cell spinophilin complex? Are additional adhesive molecules or secreted factors required to stabilize nascent dendritic cell–T cell contacts that trigger mature synapse assembly? Does mobilization of the spinophilin-linked apparatus functionally correlate with potency of response by the T cell, determine T cell fate, or differ in a compromised state, such as anergy? Which way does the information flow? Further interesting insights seem certain to follow.
Acknowledgments
The authors thank a referee for helpful citations and comments and Yair Benita for assistance with the figure.
Abbreviation used in this paper: GPCR, G protein–coupled receptor.
References
- Allen, P.B., C.C. Ouimet, and P. Greengard. 1997. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl. Acad. Sci. USA. 94:9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas, S.L., F.F. Serneo, M. Chechlacz, T.J. Deerinck, G.A. Perkins, P.B. Allen, M.H. Ellisman, and J.G. Gleeson. 2007. Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell. 129:579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, O., J.J. Unternaehrer, A. Jiang, J.-S. Shin, L. Delamarre, P. Allen, and I. Mellman. 2008. Spinophilin participates in information transfer at immunological synapses. J. Cell Biol. 181:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun, S.Y., B.P. O'Connor, A.W. Wong, H.W. van Deventer, D.J. Taxman, W. Reed, P. Li, J.S. Blum, K.P. McKinnon, and J.P. Ting. 2006. Cutting edge: rho activation and actin polarization are dependent on plexin-A1 in dendritic cells. J. Immunol. 177:4271–4275. [DOI] [PubMed] [Google Scholar]
- Feng, J., Z. Yan, A. Ferreira, K. Tomizawa, J.A. Liauw, M. Zhuo, P.B. Allen, C.C. Ouimet, and P. Greengard. 2000. Spinophilin regulates the formation and function of dendritic spines. Proc. Natl. Acad. Sci. USA. 97:9287–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui, A., S.K. Bromley, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227. [DOI] [PubMed] [Google Scholar]
- Harvey, C.D., and K. Svoboda. 2007. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 450:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker, M.S., B. Dancheck, T. Ju, R.P. Kessler, J. Hudak, A.C. Nairn, and W. Peti. 2007. Structural basis for spinophilin-neurabin receptor interaction. Biochemistry. 46:2333–2344. [DOI] [PubMed] [Google Scholar]
- Kim, E., and M. Sheng. 2004. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 5:771–781. [DOI] [PubMed] [Google Scholar]
- Ludford-Menting, M.J., J. Oliaro, F. Sacirbegovic, E.T. Cheah, N. Pedersen, S.J. Thomas, A. Pasam, R. Iazzolino, L.E. Dow, N.J. Waterhouse, et al. 2005. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 22:737–748. [DOI] [PubMed] [Google Scholar]
- Monks, C.R., B.A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86. [DOI] [PubMed] [Google Scholar]
- Satoh, A., H. Nakanishi, H. Obaishi, M. Wada, K. Takahashi, K. Satoh, K. Hirao, H. Nishioka, Y. Hata, A. Mizoguchi, and Y. Takai. 1998. Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J. Biol. Chem. 273:3470–3475. [DOI] [PubMed] [Google Scholar]
- Steinman, R.M. 2007. Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 37:S53–S60. [DOI] [PubMed] [Google Scholar]
- Steinman, R.M., and Z.A. Cohn. 1974. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J. Exp. Med. 139:380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., W. Zeng, M.S. Kim, P.B. Allen, P. Greengard, and S. Muallem. 2007. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J. 26:2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]