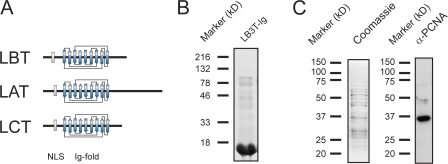
Figure 3.
X. laevis LB3T-Ig binds to PCNA in egg extracts. (A) Diagrammatic comparisons of the tail domains of LB3, LA, and LC. Note that the major conserved region among all three tail domains is the Ig-fold motif, which is ∼80% similar between human LA/C and X. laevis LB3. (B) His-tagged LB3T-Ig was bacterially expressed and purified. The purified protein was separated by SDS-PAGE and stained with Coomassie blue. (C) Purified His-tagged LB3T-Ig was bound to a Hi-Trap chelating column, X. laevis high-speed supernatant was passed over the column, and bound proteins were eluted. The fraction shown was eluted with 600 mM NaCl. The eluted proteins were separated by SDS-PAGE and either stained with Coomassie blue or transferred to nitrocellulose and probed with an antibody directed against PCNA. A band of ∼36 kD seen with Coomassie also reacts with the PCNA antibody.