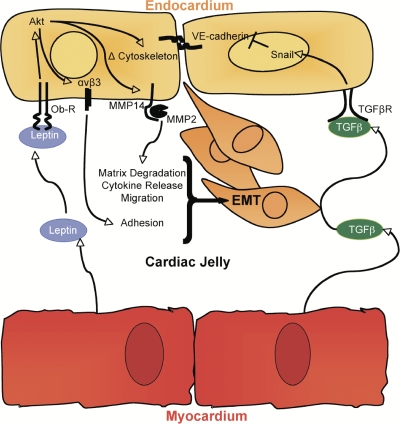
Figure 8.
Summary of leptin's roles in the endocardial cushion. The spatiotemporal pattern of leptin and leptin receptor depicts a pathway by which myocardial-derived leptin ligand engages with endocardial leptin receptors to drive EMT and migration, which is consistent with the known cell-autonomous pathway of cardiac EMT. In an ex vivo functional assay, loss of leptin abrogates adhesion, EMT, cell survival, and migration, potentially via Akt. The leptin pathway is independent of the TGF-β/Snail-driven dissolution of VE-cadherin. Interestingly, the specific effector molecules disrupted by loss of leptin in the endocardial cushion are known mediators of adhesion, migration, and differentiation. Leptin's role in adhesion of endocardial cells may occur through αvβ3 integrin receptor, whereas leptin-driven proteolytic activity and invasion of mesenchymal cells may be mediated by MMP2. Furthermore, MMPs modulate growth factor release and receptor availability and may affect morphogen gradients across the cardiac jelly and, thus, signaling cascades. The potential branch points in the leptin signaling pathway and cross talks with other pathways remain to be uncovered. Ultimately, leptin signaling affects adhesion, EMT, cell survival, and migration within the embryonic heart.