Fig. 2.
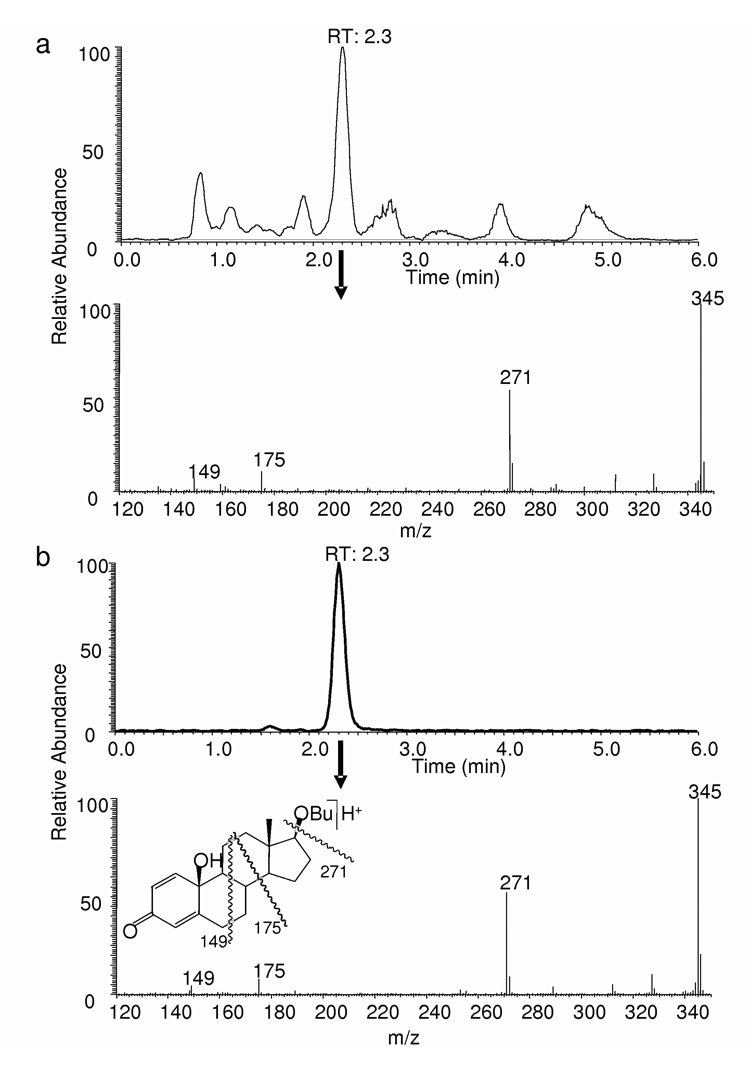
LC-MS analysis of the product formed from 17β-butoxy-1,3,5(10)-estratriene-3-ol (17OBu-E2) upon exposure to •OH generated by the Fenton reaction. (a) Extracted ion-current chromatogram (m/z 345) and APCI mass spectrum from the major LC peak of the reaction product at RT: 2.3 min; (b) Extracted ion-current chromatogram (m/z 345) and APCI mass spectra are from the major LC peak at RT: 2.3 min for synthetic 10β-hydroxy,17βbutoxyestra-1,4-dien-3,17-dione (17OBu-E2-quinol).
