FIGURE 2. The yeast kinases Kss1 and Rim11 phosphorylate c-Myc and thereby affect c-Myc protein stability.
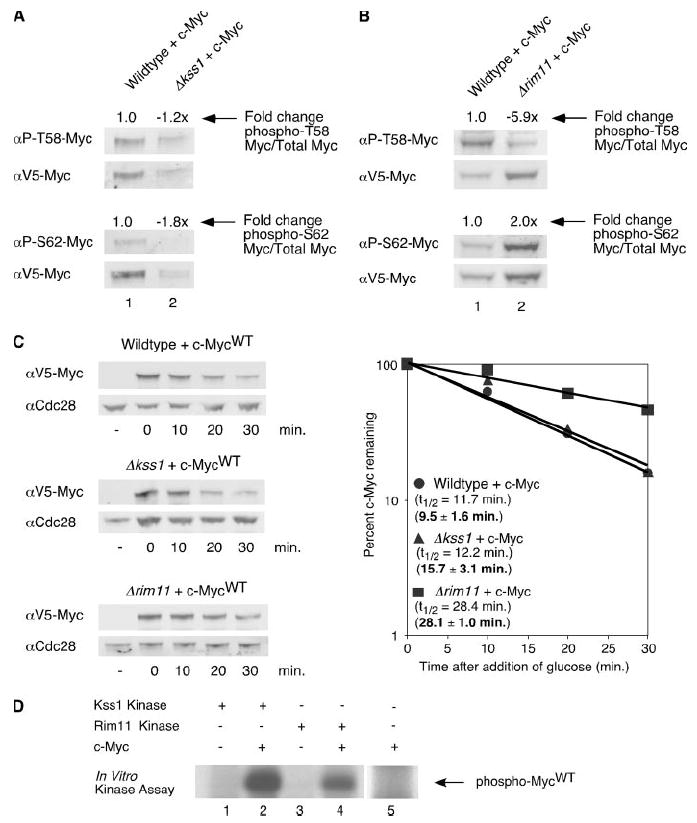
A, V5-tagged c-MycWT was expressed in the BY4741 and Δkss1 yeast strains for 1 h at 30 °C. Cells were lysed in SDS sample buffer. Equal cell numbers were analyzed by Western blotting with the indicated antibodies as described in Fig. 1A. Ratios of phosphorylated c-MycWT to total c-MycWT were calculated. -Fold change of ratios in the Δkss1 strain compared with the BY4741 strain are shown. B, V5-tagged c-MycWT was expressed in the BY4741 and Δrim11 yeast strains for 1 h at 30 °C. Cells were lysed in SDS sample buffer. Equal cell numbers were analyzed by Western blotting as described in Fig. 1A. Ratios of phosphorylated c-MycWT to total c-MycWT were calculated. -Fold change of ratios in the Δrim11 strain compared with the BY4741 strain are shown. C, V5-tagged c-MycWT expression was induced in the BY4741, Δkss1, or Δrim11 strain for 1 h at 30 °C. After the addition of glucose, cells were harvested at the time points indicated, and cells were lysed in SDS sample buffer. Western blotting and quantitation were performed as described in Fig. 1B. Experiments were repeated three or more times, and representative data are shown. Mean half-lives ± S.D. are indicated in bold. D, lysates from Δkss1 or Δrim11 strains expressing or not expressing V5-His6-tagged c-Myc protein were incubated with nickel-agarose to purify c-Myc. Kss1 and Rim11 kinases were purified from yeast strains with knocked-in TAP tags using calmodulin purification as described under “Materials and Methods”. c-Myc or lysates not expressing c-Myc were eluted from the nickel-agarose and then incubated with either the immobilized TAP-Kss1 or TAP-Rim11 kinases in the presence of 32P-labeled ATP (lanes 1– 4). As an additional control, c-Myc protein was incubated with 32P-labeled ATP in the absence of either TAP-tagged kinase (lane 5). Representative results are shown. αP-S62, α-Ser(P)-62; αP-T58, α-Thr(P)-58.
