Abstract
Immunoglobulin A (IgA)-nephropathy (IgAN) is the most common primary renal glomerular disease in the world that has no effective treatment. High levels of circulating IgA-fibronectin (Fn) complexes, characteristically found in IgAN patients, are suggested to cause abnormal deposition of IgA and Fn in the renal glomeruli of these patients causing renal failure. We previously reported that binding of Fn to uteroglobin (UG), a multifunctional anti-inflammatory protein, inhibits Fn-IgA heteromerization. However, the specific site of Fn-UG interaction until now remained unidentified. We report here that UG interacts with the heparin-binding site of Fn and propose that small molecules competing for interaction with this site may reduce the level of circulsating Fn-IgA complexes in IgAN.
1. Introduction
Uteroglobin (UG), also known as Clara cell 10 kDa (CC10) protein, is the founding member of a newly recognized superfamily of proteins called Secretoglobin (1). It is a steroid-inducible, multifunctional, secreted protein with potent anti-inflammatory and anti-chemotactic properties (2). We previously reported that UG-knockout (UG-KO) mice(3) develop abnormal deposition of immunoglobulin A (IgA) and fibronectin (Fn) in the renal glomeruli, reminiscent of the pathologic finding characteristic of human IgA-nephropathy (IgAN)(4). IgAN is worldwide the most common primary glomerular disease that has no effective treatment (5). It has been reported that in patients with IgAN high levels of circulating Fn-IgA complexes are found and these complexes are suggested to play critical roles in the abnormal deposition of IgA and Fn in the renal glomeruli leding to IgAN pathogenesis (6). Consistent with this finding, we uncovered that in the UG-KO mice serum levels of Fn-IgA complexes are also present (4). More importantly, we found that treatment of these mice with purified recombinant human UG (hUG) prevents abnormal glomerular deposition of Fn and IgA (4). However, the specific site of Fn on which UG interacts and the molecular mechanism(s) by which UG suppresses the formation of Fn-IgA heteromers remain unclear.
Fibronectin (Fn) is a large adhesive glycoprotein composed of two nearly identical subunits of 220 kDa (reviewed in 7, 8). Each of these subunits contains a series of functional binding domains on which several proteins have been reported to interact (9). Fn is essential for normal growth and development (10) and it plays critical roles in cell attachment, movement, wound healing and cancer (for review see 11). Fn is detectable as a soluble glycoprotein in blood and other body fluids and an insoluble form constitutes a major extracellular matrix protein in tissues.
We previously reported that UG binds Fn and prevents abnormal glomerular deposition of Fn and IgA (4), a characteristic pathologic finding in human IgA-nephropathy (IgAN). We have also reported that UG binds Fn and most likely suppresses the formation of Fn-IgA heteromers (). However, the specific site on Fn with which UG interacts and prevents Fn-IgA heteromerizaton, until now, remained unclear. The identification of this site on Fn may yield valuable information for the development of small molecules that functionally mimic UG and prevent Fn-IgA heteromerization. Thus, this site may be a potential target for developing novel approaches to reduce the level of circulating Fn-IgA complexes in IgAN. Accordingly, we carried out experiments to identify and characterize the specific site(s) on Fn with which UG interacts forming Fn-UG heteromes.
2. Materials and Methods
2.1. Reagents
Fn and its peptide fragments were purchased from Sigma and Invitrogen, respectively. Purified secretory IgA from human colostrum and BSA were purchased from Sigma (St.Louis, MO). Labeling of recombinant human UG (hUG) was performed by Amersham Radiochemicals.
2.2. Radioiodination of Recombinant IgA
This purified IgA (25μg) was radioiodinated by using Na125I (2mCi; carrier free; 1Ci = 37 GBq) and Iodo-Beads. The reaction was carried out in 130 μl of PBS; pH 7.4, at 25°C for 10 min. 125I-IgA was purified by Sephdex G-25 spun-column chromatography (1200 X g for 4 min). The column was prewashed with 0.05% bovine serum albumin (BSA) in PBS. The specific activity of purified carrier-free 125I-human IgA was 20μCi/μmole.
2.3.Preparation of pure recombinant hUG and Radiolabeled of hUG
Recombinant hUG was expressed in Escherichia coli and purified to homogeneity as previously reported (12). This protein (25 μg) was radioiodinated using sodium [125I] (2mCi; carrier free; 1Ci = 37 GBq) and IODO-BEADS. The reaction was carried out in 130 μl of phosphate-buffered saline (PBS; pH 7.4) at 25°C for 10 min, and was [125I]-hUG was purified by Sephdex G-25 spun-column chromatography (1200 x g for 4 min). The column was pre washed with 0.05% bovine serum albumin (BSA) in PBS. The specific activity of purified carrier-free 125I-hUG was 25μCi/μmole.
2.4.Ligand affinity chromatography
Fibronectin and and its different fragments were purchased from Sigma and Gibco BRL for the preparation of fibronectin Sepharose affinity column. Fibronectin and its fragments were dialyzed overnight at 4°C against 0.1 M NaHCO3 buffer, pH 8.3, containing 0.5 M NaCl (coupling buffer). Fibronectin was then coupled to cyanogen bromide activated Sepharose-4-B (Pharmica Fine Chemicals, Piscataway, NJ) according to the manufacturer’s instructions. Briefly, fibronectin and its fragments were incubated overnight at 4°C with 0.3 g cyanogen bromide-activated sepharose, which was pre-washed 3 times with 1mM HCl. Active groups were blocked with 0.2 M glycine for 20 hours at 4°C. Excess ligand was removed by alternatively washing the resin with the coupling buffer and 0.1 M Sodium Acetate buffer, pH 4.0, containing 0.5 M NaCl. 100 μl of each mixture containing Fn or its fragments supported with Sepharose-4-B were incubated with 3H-hUG (2μCi) under physiological condition in binding buffer in Hanks’ balance salt solution (HBBS), pH 7.4 containing 0.1 % bovine serum albumin in the presence and absence of excess unlabeled hUG at room temperature for 1 h. The reaction was stopped by rapid removal of unbound 3H-hUG and the matrix was washed three times with PBS, pH 7.4. The radioactivity was taken with radioactive counter.
2.5. Binding of hUG and IgA with Fn affinity column
Binding reactions of hUG or IgA with Fibronectin and its fragments containing different binding domain in solution were carried out at room temperature by mixing 0.5 to 1.5 pmol of the 125I-ligand ( hUG or IgA; 5–15 X 104 cpm/pmol) with 50 pmol biotinylated Fn and its fragments in 300 μl of 50mM sodium phosphate, pH 7.4, containing 100 mM NaCl (PBS) in absence and presence of varying concentration of cold ligand during competition studies. After 30 min. of incubation, 1 mg/ml bis(sulfosuccinimidyl) suberate (BS3 ; Pierce) was added in the reaction mixture and after another 30 min of incubation, the cross-linker was inactivated by adding 30 μl of 2M glycine, pH 7.4. at room temperature for 30 min. Thereafter, 30 μl of 40 mM BSA was added. From each reaction vial, 300 μl was transferred to a new vial containing 10 μl of prepacked streptavidin-conjugated agarose beads (1.5 mg streptavidin /ml; Pierce) and 50 μl of packed Sepharose CL-4B (Pharmacia Biotech Inc.). The Sepharose and agarose beads were preblocked with 2 mg /ml BSA in PBS to reduce the nonspecific binding. Binding to the beads was carried out at 4’C over night on an end-over-end rotator. The reaction was stopped by rapid removal of unbound radioactive material and the reaction bead was washed four times with PBS, pH 7.4 containing 0.05 % Tween 20. The specific binding was calculated by subtracting the non specific binding from the total binding. The binding was analyzed by Scatchard plot using the Ligand computer program.
2.6. In vitro binding of hUG and Fn
Binding studies using recombinant hUG and purified Fn binding were also carried out by incubating a mixture of 10nM purified [3H]-hUG (Amersham Radiochemicals) and Fn (100μg/ml) containing varying concentrations of unlabeled hUG (0–50 nM) in 50mM sodium phosphate buffer containing150 mM KCl, pH 7.5, was incubated for 60 min at 4’C in the presence of 10mM of the crosslinking agen, DSS (Pierce). The reaction was stopped by adding 1M Tris-HCl, pH 6.8 to 1/10 the volume of the reaction mixture and the products were resolved electrophoresis using a 4–20% SDS gel followed by autoradiography using a Cyclone phosphorimager (Packard).
2.7. Statistical analysis
Statistical analyses of the data were performed using Graph Pad Prism V4.0 software (Graph Pad Software, Inc., San Diego, CA) and Minitab Version 13 (Minitab, Inc., State College, PA). p-values ≤ 0.05 were considered as statistically significant. All experiments were repeated three times.
3. Results and Discussion
3.1. Recombinant human UG binds to the heparin-binding site of fibronectin
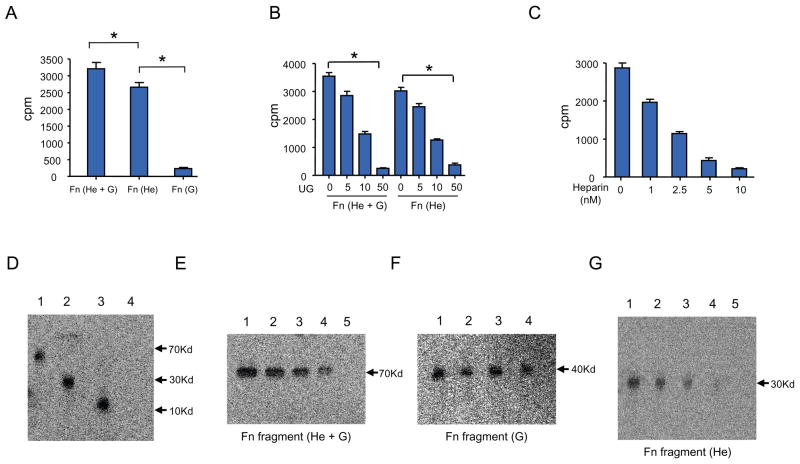
Fibronectin consists of type I, type II and type III repeats, and the variable connecting sequence with several binding domains including those that specifically bind heparin- and gelatin. It has been reported that each of these binding domains has specific functional activity (13). We first sought to identify and characterize the specific site(s) on Fn with which UG interacts. Accordingly, we first carried out binding studies using [3H] recombinant human UG (hUG) and synthetic peptides corresponding to various fragments of Fn containing the binding sites for heparin+gelatin (He+G), heparin (He) only or gelatin (G) only. The results show that recombinant hUG interacts with the Fn fragments that contain He+G and He-binding sites but does not interact with the Fn fragment containing G-binding site only (Fig. 1A). In order to determine which of the two sites [i.e. heparin- (He) or gelatin-(G) binding sites] specifically interacts with hUG in further detail, we carried out binding studies using Fn fragments, He+G and He by incubating each of these fragments with equal amounts of 3H-hUG followed by addition of increasing concentrations of non-radioactive hUG for competition. Our results show that 3H-UG bound to both Fn fragnments is displaced by unlabeled hUG in a dose-dependent manner (Fig. 1B). These results show that UG binds to the He-binding site of Fn with high specificity. To further confirm whether hUG specifically interacts with the He-binding site of Fn, we incubated a fixed concentrations Fn and 3H-hUG together with increasing concentrations of heparin in a competition assay. The results show that non-radioactive heparin displaces 3H-hUG bound to He containing fragment of Fn in a dose-dependent manner (Fig. 1C). These results strongly suggest that hUG specifically binds to the heparin-binding site of Fn.
Figure 1.
A. Binding of hUG with Fn and its fragments containing different binding domain. One hundred microliter of mixtures containing Fn or its different fragments (Pharmica Fine Chemicals, Piscataway, NJ) containing each of the following binding sites: (He + G)-, (He)- or (G) were incubated with 3H-hUG (2μCi) in the binding buffer (Hanks’ Balance Salt Solution, pH 7.4 containing 0.1 % bovine serum albumin) at the room temperature for 1 h. The reaction was stopped by washing three times with 1x PBS, pH 7.4 to remove 3H-hUG. The data are presented as the mean (n=3 experiments) + SD. B. Binding of 3H-hUG with the Fn fragment containing (He+G) and (He) sites. Binding studies were performed as described with varying concentrations of unlabeled hUG (0 to 50 nM) at room temperature for 1 h. The data are presented as the mean (n=3 experiments) + SD. C. Binding specificity of hUG with the Fn fragment containing the (He)-binding site. Binding studies were carried out as described except that non-radioactive heparin in varying concentrations (0 to 50 nM) were used for competition with 3H-hUG. The data are from three experiments, and each data point represents the mean of triplicate determinations. D. Affinity crosslinking studies. Affinity crosslinking studies were carried out as described under Material and Methods. Binding and crosslinking of 3H-hUG-Fn or its fragments containing different binding domains are readily detectable. Lane 1:He + G; Lane 2:He only; Lane 3: 3H-hUG control; Lane 4: Myoglobin (Myg) as a non-specific control for hUG. E. Determination of specificity of 3H-hUG-binding to (He + G)-binding site of Fn. (He +G) containing fragment of Fn was incubated with a fixed concentration of 3H-hUG and competed with varying concentrations of non-radioactive hUG. Lane 1: control without non-radioactive hUG, Lane 2: 2 nM hUG; Lane 3: 5 nM hUG; Lane 4: 10 nM hUG; Lane 5: 25nM hUG. F. Non-specific binding of Fn fragment containing (G)-binding site only. Lane 1: Control without hUG, Lane 2: 5 nM hUG; Lane 3: 10 nM hUG; Lane 4: 25 nM hUG. G. Specificity of 3H-hUG binding with the (He)-binding site of Fn. Lane 1: 2 nM hUG; Lane 2: 5 nM hUG; Lane 3: 10 nM hUG; Lane 4: 25nM hUG; Lane 5: 50 nM hUG. (He)=Fn fragment containing the heparin binding site; (G)=Fn fragment containing the gelatin-binding site; hUG= human uteroglobin.
3.2. Binding of UG to the heparin-binding site of Fn is highly specific
To further confirm the specificity of hUG binding to the heparin-binding site of Fn, we incubated total Fn or its fragments containing either He- or G-binding sites with a specific concentration of 3H-hUG together with increasing concentrations of non-radioactive hUG. The protein mixture was resolved by SDS-PAGE under non-denaturing and non-reducing conditions and radioactive protein bands were detected using a phosphorimager (Packard). The results show that radioactive hUG bound to Fn fragment containing He-or He+G-binding sites (Fig. 1D). Most importantly, non-radioactive hUG displaced 3H-hUG from the Fn fragment containing the He-binding site only in a dose-dependent manner (Fig. 1E), while it failed to displace radioactive hUG from the Fn-peptide that contained only the G-binding site (Fig. 1F). Taken together, these results demonstrate that hUG binds to the heparin-binding site of Fn with high specificity.
To gather further proof that the interaction of hUG with Fn indeed requires the heparin-binding site of Fn, we performed affinity-crosslinking studies using 3H-hUG and non-radioactive Fn as well as Fn-peptide fragment containing only the (He)-binding site in the presence or absence of varying concentrations of non-radioactive hUG. Disuccinimidylsuberate (DSS), a crosslinking agent, was added to the mixture. The protein mixture was resolved by SDS-PAGE under denaturing and reducing conditions and the radioactive protein bands were detected by using a phosphorimager. 3H-hUG bound to the Fn fragment containing the (He)-binding site and this binding is displaced by non-radioactive hUG in a dose-dependent manner (Fig. 1G, lanes 1–5).
3.3. Fibronectin binds to UG with higher affinity that IgA
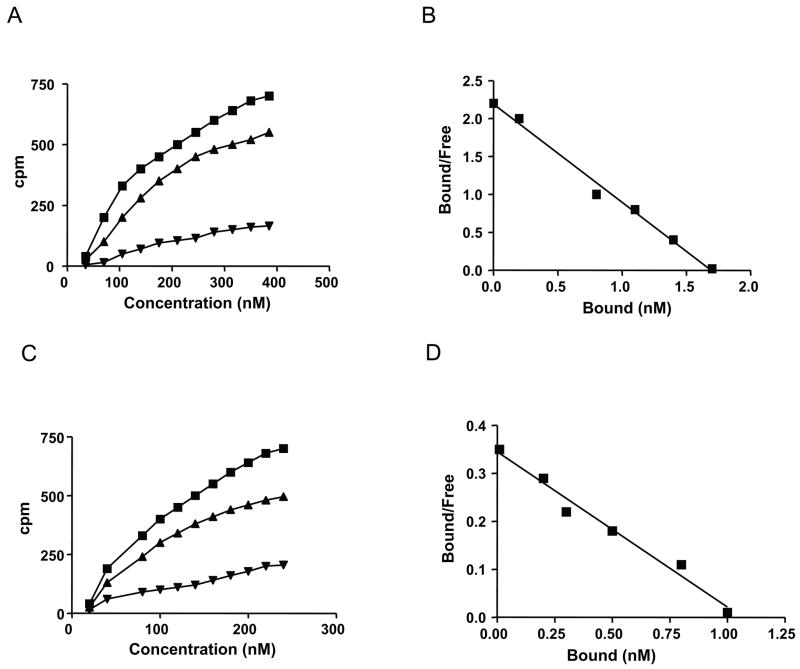
We previously reported that Fn-hUG interaction prevents Fn-IgA heteromerization, reported to be critical for abnormal accumulation of IgA in the renal glomeruli of IgAN patients (6) as well as in those of the UG-KO mice (4). The results of our present study show that hUG specifically binds Fn with its carboxy-terminal heparin-binding domain but not with the gelatin binding site. Since hUG prevents Fn-IgA heteromerization, we sought to determine the affinities of Fn for binding to hUG and to IgA. Accordingly, we conducted binding studies using 125I-UG or 125I-IgA and non-radioactive Fn in presence of increasing concentrations of either non-radioactive hUG or IgA. The disassociation constants (Kds) for Fn binding to IgA and Fn binding to hUG were determined. The specific binding of 125I-UG to Fn was saturable (Fig. 2A) and specific. Further, the Scatchard analysis of these data show that there is a single high-affinity binding-site for hUG on Fn. The calculated apparent dissociation constant (Kd) for binding of hUG to Fn is 15 nM (Fig. 2B). Similar experiments conducted with fixed concentration of 125I-IgA binding to Fn and increasing concentrations of unlabeled IgA show that binding of 125I-IgA to Fn is also saturable (Fig. 2C) with a dissociation constant (Kd) of 65 nM (Fig. 2D). Taken together, these results raise the possibility that the high-affinity binding of hUG to Fn (Kd = 15 nM) is effective in preventing the Fn-IgA complex formation, reported to cause abnormal accumulation of IgA and Fn in renal glomeruli leading to IgAN.
Figure 2.
A. Saturation isotherm and Scatchard analysis of 125I-UG binding to Fn. Binding isotherms of hUG to Fn were performed by incubating increasing concentrations of 125I-UG. Specific binding (■) was calculated by subtracting nonspecific binding (▲), measured as the residual binding in the presence of hUG, from the total binding (■). The data shown are the mean of triplicate measurements±SD. B. Scatchard plots of hUG binding to Fn. The apparent dissociation constant (Kd ) estimated from the slope of the line was 15 nM ± 2.6 nM (n = 3). C. Binding isotherms of IgA to Fn. Binding isotherms of IgA to Fn were performed by incubating increasing concentration of 125I-IgA. Specific binding (■) was calculated by subtracting nonspecific binding (▲), measured as the residual binding in the presence of IgA, from the total binding (■). The data shown are the mean of triplicate measurements. D. Scatchard plots of IgA binding to Fn. The apparent dissociation constant (Kd) estimated from the slope of the line was 65 nM ± 5.2 nM (n = 3).
Several regions of Fn are involved in supporting specific biological functions such as cell adhesion, spreading and migration. In addition, Fn is a major constituent of the extracellular matrix. In pathologic conditions such as IgAN, abnormal deposition of Fn and IgA in the renal glomeruli resulting from excessive circulating Fn-IgA heteromers appears critical for IgAN pathogenesis (6). Thus, inhibition of Fn-IgA heteromerization may facilitate the development of novel therapeutic strategies for IgAN. In this study, we have demonstrated that the interaction of Fn with UG occurs on its heparin binding site. The identification of this UG binding site on Fn not only explains the molecular mechanism by which UG prevents abnormal deposition of IgA and Fn in the glomeruli but also demonstrate that this region of Fn is a potential target for the development of small molecules that prevent Fn-IgA heteromerization and prevent IgAN.
It has been reported that recombinant human UG prevents adhesion and migration of human endothelial cells in vitro by its specific interaction with fibronectin (14). However, the specific region(s) of the Fn molecule with which UG interacts and exerts its biological functions until now remained undefined. From the perspective of IgAN, identification of this site may allow development of small molecules that competes for interaction with this site and prevents the formation of Fn-IgA heteromers reported to be associated with IgAN (6, 15). There are three different repeating sequences in each Fn subunit, type I, II and III. Incorporation of dimeric Fn into extracellular matrix (ECM) requires consecutive binding interactions with itself, with integrin receptors, and with various components of the ECM such as type I collagen (reviewed in 16, 17). There are also two heparin binding sites (Hep I and Hep II) in the amino- and carboxy terminal, respectively, of Fn. The heparin-binding domain at the COOH-terminal of Fn binds CD44 (18), a high affinity receptor for hyaluronan (19). Amino-terminal heparin binding fragments of Fn stimulates proteoglycan breakdown (19) and release catabolic cytokines (20). It has been reported that the 40kDa COOH-terminal heparin-binding fragment of Fn binds to CD44 and regulates MMP gene expression (21). By competing with CD44 for interaction with the heparin-binding site of Fn, hUG may prevent the release the cytokines that are proinflammatory and help to maintain homeostasis. This may be another mechanism by which UG exerts its anti-inflammatory effects (2).
In summary, we have identified the specific site on the Fn molecule with which UG interacts and suppresses the formation of Fn-IgA complexes, which are found at a high level in the circulation of IgAN patients and thought to contribute of abnormal deposition of IgA and Fn in the renal glomeruli of the IgAN patients leading to renal failure. Thus, our results identify a potential drug target for IgAN.
Acknowledgments
We thank Drs. J.Y. Chou and I. Owens for critical review of the manuscript and helpful suggestions. This research was supported in full by the intramural NICHD, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukherjee AB, Chilton BS. The uteroglobin/Clara cell protein family. Nomenclature Committee Report. Ann NY Acad Sci. 2000;293:348–356. doi: 10.1111/j.1749-6632.2000.tb05549.x. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocrine Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Kundu GC, Yuan CJ, Ward JM, Lee EJ, DeMayo F, Westphal H, Mukherjee AB. Severe fibronectin-deposit renal glomerular disease in mice lacking uteroglobin. Science. 1997;276:1408–1412. doi: 10.1126/science.276.5317.1408. [DOI] [PubMed] [Google Scholar]
- 4.Zheng F, Kundu GC, Zhang Z, Ward J, DeMayo F, Mukherjee AB. Uteroglobin is essential in preventing immunoglobulin A nephropathy in mice. Nat Med. 1999;5:1018–1025. doi: 10.1038/12458. [DOI] [PubMed] [Google Scholar]
- 5.Hsu SI. The molecular pathogenesis and experimental therapy of IgA nephropathy: recent advances and future directions. Curr Mol Med. 2001;1:183–196. doi: 10.2174/1566524013363852. [DOI] [PubMed] [Google Scholar]
- 6.Cederholm B, Wieslander J, Bygren P, Heinegard D. Circulating complexes containing IgA and fibronectin in patients with primary IgA nephropathy. Proc Natl Acad Sci U S A. 1988;85:4865–4868. doi: 10.1073/pnas.85.13.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hynes RO. Fibronectins: a family of complex and versatile adhesive glycoproteins derived from a single gene. Harvey Lect. 1985–1986;81:133–152. [PubMed] [Google Scholar]
- 8.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 9.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 10.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO, Schwarzbauer JE, Tamkun JW. Fibronectin: a versatile gene for a versatile protein. Ciba Found Symp. 1984;108:75–92. doi: 10.1002/9780470720899.ch6. [DOI] [PubMed] [Google Scholar]
- 12.Mantile G, Miele L, Cordella-Miele E, Singh G, Katyal SL, Mukherjee AB. Human Clara cell 10-kDa protein is the counterpart of rabbit uteroglobin. J Biol Chem. 1993;268:20343–20351. [PubMed] [Google Scholar]
- 13.Hayashi M, Schlesinger DH, Kennedy DW, Yamada KM. Isolation and characterization of a heparin-binding domain of cellular fibronectin. J Biol Chem. 1980;255:10017–10020. [PubMed] [Google Scholar]
- 14.Antico G, Lingen MW, Sassano A, Melby J, Welch RW, Fiore S, Pilon AL, Miele L. Recombinant human uteroglobin/CC10 inhibits the adhesion and migration of primary human endothelial cells via specific and saturable binding to fibronectin. J Cell Physiol. 2006;207:553–561. doi: 10.1002/jcp.20604. [DOI] [PubMed] [Google Scholar]
- 15.Coppo R, Amore A, Gianoglio B, Porcellini MG, Peruzzi L, Gusmano R, Giani M, Sereni F, Gianviti A, Rizzoni G, et al. Macromolecular IgA and abnormal IgA reactivity in sera from children with IgA nephropathy. Italian Collaborative Paediatric IgA Nephropathy Study. Clin Nephrol. 1995;43:1–13. [PubMed] [Google Scholar]
- 16.Mosher DF. Assembly of fibronectin into extracellular matrix. Curr Opin Struct Biol. 1993;3:214–222. [Google Scholar]
- 17.Schwartz MA, Schaller MD, Ginnsberg MH. Integrins: Emerging Paradigms of Signal Transduction. Ann Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 18.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aruffo A, Stamenkovic I, Melnick M, Uderhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda T, Poole AR. A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheumatism. 2002;4:138–148. doi: 10.1002/1529-0131(200201)46:1<138::AID-ART10051>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda T, Poole AR, Shimizu M, Nakagawa T, Julovi SM, Tamamura H, Fujii N, Nakamura T. Involvement of CD44 in induction of matrix metalloproteinases by a COOH-terminal heparin-binding fragment of fibronectin in human articular cartilage in culture. Arthritis Rheumatism. 2003;48:1271–1280. doi: 10.1002/art.10951. [DOI] [PubMed] [Google Scholar]