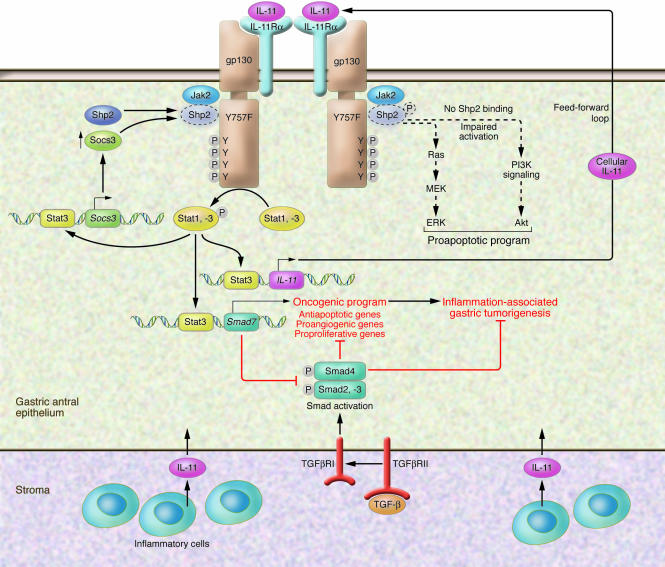
Figure 1. Mechanism of IL-11–mediated induction of gp130 and gastric tumorigenesis.
Schematic of mucosal changes that occur during chronic gastric inflammation, in which IL-11 is one of the cytokines produced. IL-11 binds to the IL-11 receptor α (IL-11Rα) subunit that heterodimerizes with the gp130 homodimeric receptor. IL-11 is presumably produced by both the epithelium and inflammatory cells and binds to the gp130 receptor also located on epithelial cells in the gastric antrum. As described in the current study by Ernst et al. (12), in the presence of the Y757F mutation in the gp130 receptor, SRC homology 2 domain–containing (SH2-containing) tyrosine phosphatase (Shp2) phosphorylation and subsequent activation of the proapoptotic Ras/Erk and PI3K/AKT pathways do not occur. Instead, phosphorylation of the four C-terminal tyrosine residues in the gp130 receptor, in the absence of phosphorylation of Tyr757, results in Stat activation. Stat3 is activated to a greater extent than Stat1. Suppressor of cytokine signaling 3 (Socs3) is a downstream target of Stat3 and competes with Shp2 for docking at Tyr757. Therefore, epithelial Socs3 levels in the cells rise, but they cannot bind gp130 receptor and prevent its signaling. In addition, Stat activation induces the expression of the inhibitory protein Smad7, which blocks TGF-β–activated Smad signaling. In this way, Stat3 hyperactivity induced by the IL-11 proinflammatory cytokine can suppress the cytostatic effect of the stroma on cell proliferation (22). Moreover, Stat3 also induces epithelial cell expression of IL-11, setting a feed-forward mechanism that fuels persistent cellular proliferation. Collectively, these signaling events promote an oncogenic program in which the expression of antiapoptotic, proangiogenic, and proproliferative genes results in inflammation-associated gastric tumorigenesis. F, phenylalanine; P, phosphate; TGFβR, TGF-β receptor; Y, tyrosine.