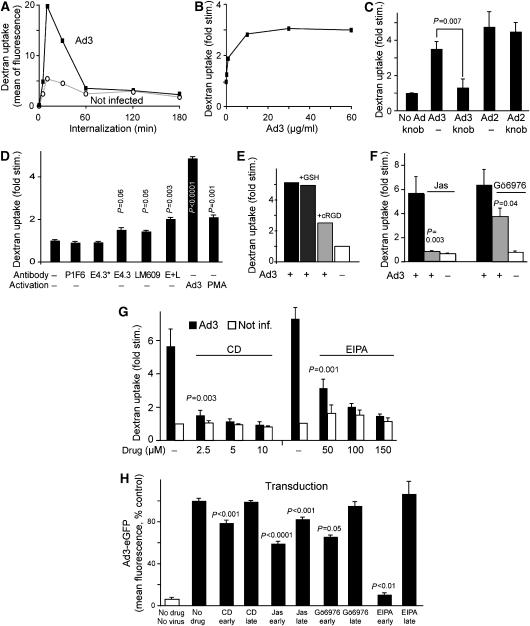
Figure 3.
CD46-, integrin-, F-actin-, PKC-, and EIPA-dependent stimulation of fluid-phase endocytosis by Ad3. (A) Ad3 transiently stimulates dextran uptake. HeLa-ATCC cells were incubated with Ad3 (5 μg/ml, equivalent to 2000 particles bound per cell) in the cold for 60 min, or noninfected, warmed for 0, 5, 10, 30, 60, 120, or 180 min, pulsed with dextran—FITC, and analysed by flow cytometry as described in Materials and methods. One out of three representative experiments is shown. (B) Dose dependence of fluid-phase uptake stimulation expressed as fold stimulation over noninfected cells. HeLa cells were incubated with different amounts of Ad3 (0, 0.5, 1, 10, 30, and 60 μg/ml) in the cold, warmed for 5 min, pulsed with dextran–FITC for 5 min, washed, and analysed by flow cytometry. One out of two similar experiments is shown. (C) Specificity of Ad3-stimulated fluid-phase endocytosis. HeLa cells were incubated with Ad3 or Ad2 (5 μg/ml) in the presence or absence of Ad3 fibre knob (5 μg/ml) and analysed for dextran–FITC stimulation as described above. (D) Fluid-phase stimulation by anti-CD46 and anti-integrin antibodies. HeLa cells were incubated with 4 μg/ml anti-CD46 antibody E4.3, or 0.8 μg/ml E4.3 (E4.3*), anti-αv β5 integrin (P1F6), anti-αv β3 (LM609), or a combination of E4.3 plus LM609 (E+L, 4 μg/ml each) in cold RPMI medium for 1 h, washed, and incubated with goat anti-mouse IgG antibodies (10 μg/ml), Ad3, or the phorbol ester PMA (Meier et al, 2002) on ice for 30 min. They were then warmed in the presence of dextran–FITC for 10 min and analysed by flow cytometry. The experiment was performed in triplicate and repeated once. (E–G) Measurement of Ad3-induced dextran–FITC uptake 10 min p.i. in the presence or absence of cyclic RGD peptides (0.1 mM; Meier et al, 2002), jasplakinolide (Jas, 40 nM), the PKC inhibitor Gö6976 (1 μM), or different concentrations of cytochalasin D (CD) or EIPA. (H) Ad3-eGFP transduction (1000 viral particles/cell) of HeLa cells pretreated with CD, Jas, Gö6976, or EIPA. Gö6976 and EIPA were present during 1 h of warm infection and were then washed off (early), or present till 120–180 min p.i. (late), followed by flow cytometry of eGFP 6 h p.i. CD and Jas were present during the entire incubation time (early) or added 1 h after warming (late). Experiments were performed at least twice with triplicate samples.