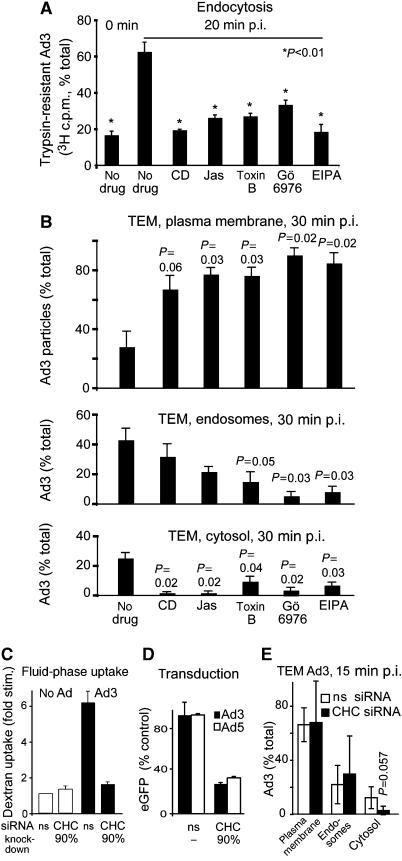
Figure 4.
Ad3 endocytosis and endosomal escape require F-actin, Rho GTPases, PKC, the sodium–proton exchanger 1, and clathrin. (A) Endocytosis of Ad3 measured by trypsin sensitivity of cell-surface-localized virus. HeLa-ATCC cells were pretreated with cytochalasin D (CD, 5 μM), jasplakinolide (Jas, 0.3 μM), Clostridium difficile toxin B (toxB, 0.3 μg/ml) (Aktories, 1997), Gö6976 (1 μM), or EIPA (100 μM) in growth medium for 30 min, incubated with [3H]thymidine-labelled Ad3 (50 000 c.p.m.) in the cold for 1 h, washed and internalized at 37°C for 20 min, washed with cold medium, and treated with trypsin (2 mg/ml) at 4°C for 1 h. Cells were pelleted by centrifugation at 500 g and the supernatants and cell pellets were analysed by liquid scintillation counting (fraction of total, 100% equivalent to 2000 c.p.m.). (B) Analysis of subcellular localization of Ad3 particles by transmission EM. HeLa cells were pretreated with drugs as described in panel A, incubated with Ad3 (30 μg/ml) in the cold, washed with binding medium, internalized in drug-containing medium for 30 min, and fixed for ultrathin-section EM analyses. Viral particles were quantified at the plasma membrane, in endosomes, and in the cytosol as described (Meier et al, 2005). The total number of particles blindly analysed for each condition was 200–300 in 6–9 different cells. For representative images, see Supplementary Figure 2. (C–E) Ad3-stimulated dextran uptake and infection required CHC. Uptake measurements of dextran–FITC in normal HeLa-ATCC cells or cells transfected with nonsilencing siRNA (ns) or siRNA against CHC (CHC, double transfection, 72 h), eGFP transduction measurements, as well as EM analyses were performed as described.