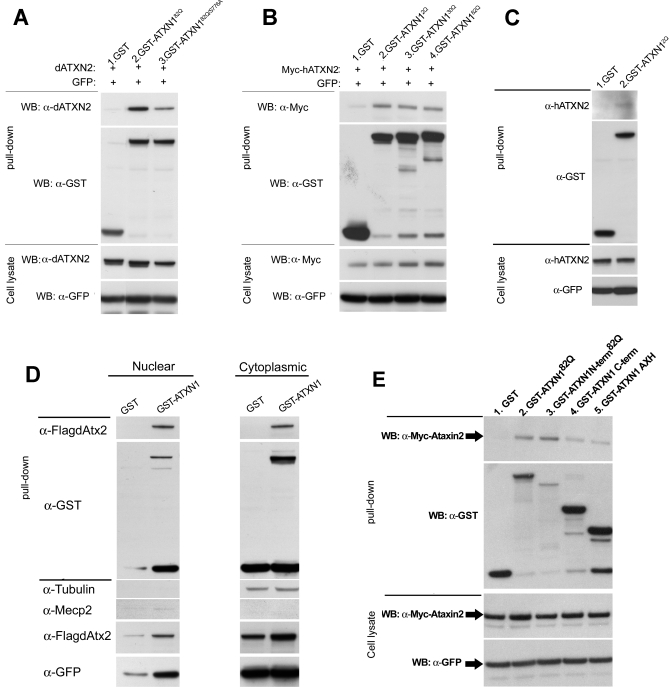
Figure 4. The Ataxin-1[82Q] and Ataxin-2 Proteins Physically Interact.
(A) GST Co-AP pull-down experiments between dAtx2 and GST-constructs carrying human Ataxin-1[82Q] or Ataxin-1[82Q] with S776A mutation.
(B) GST Co-AP experiments between human Ataxin-2 (Myc-hATXN2) and GST-Ataxin-1 with different polyglutamine lengths.
(C) Interaction between GST-Ataxin-1[2Q] and endogenous hATXN2 in cell culture.
(D) Co-AP pull down experiments between GST-Ataxin-1 and Flag-dAtx2 after nuclear/cytoplasmic fractionation. Tubulin is used as cytoplasmic marker and Mecp2 as nuclear marker.
(E) Comparative analysis of the interaction of hAtaxin-2 with different domains of the Ataxin-1 protein. Lane-1, GST alone does not pull down Myc-hAtaxin2. Lane-2, GST-ATXN182Q pulls down Myc-hAtaxin2. Lane-3 expanded N-terminal Ataxin-1 (GST-ATXN1N-term82Q) lacking the AXH domain (aa# 1–575) also pulls down Myc-hAtaxin-2 with high affinity. Lanes-4 and 5 show that both the C-terminus portion of Ataxin-1 containing the AXH domain (aa# 529–816; lane-4) or the AXH domain alone (aa# 558–700; lane-5) pull down Myc-hAtaxin2. These interactions are weaker than that of the N-terminus part (compare lanes 4 and 5 with lane 3). Cell lysates for co-AP were ran through Glutathione conjugated beads, and blots stained with anti-GST or anti-Myc. Immunoblots for dAtx2, Myc-hAtaxin2 and GFP carried out on cell lysates before the co-AP pull-down reveal similar levels of both proteins between samples.