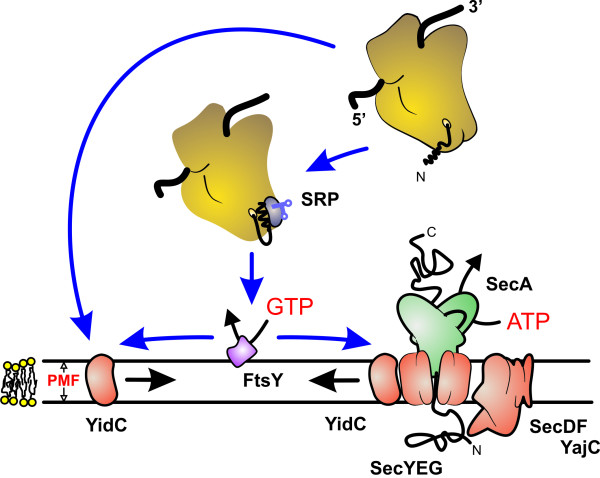
Figure 3.
Scheme of membrane protein targeting and insertion by the Sec translocase and YidC. The bacterial Sec translocase is a protein complex in the cytoplasmic membrane, which comprises a peripheral motor domain SecA, the protein-conducting channel SecYEG, and the accessory proteins SecDF(yajC) and YidC. Membrane proteins are cotranslationally targeted to the Sec translocase as ribosome-bound nascent chains by the SRP and the SRP-receptor FtsY. FtsY associates with the SecY subunit of the Sec translocase, and associates with SRP in a GTP-dependent fashion. GTP hydrolysis at FtsY and SRP effects the release of the ribosome-nascent chain complex from SRP to the SecY subunit of the Sec translocase. Next, chain elongation at the ribosome is directly coupled to the SecY-mediated insertion of the nascent membrane protein into the cytoplasmic membrane. During membrane insertion, newly synthesized transmembrane segments of nascent membrane proteins contact YidC, which may facilitate the lateral release of these hydrophobic segments into the lipid bilayer and/or assist in their folding and assembly. Translocation of large polar extracellular regions through the SecYEG translocation pore is effected by SecA at the expense of ATP. YidC also acts as a Sec-independent membrane protein insertase for a number of small membrane proteins. These proteins are either targeted directly to YidC, or possibly utilize SRP and FtsY for targeting. How SRP discriminates between SecYEG- and YidC-dependent targeting of nascent membrane proteins is unknown. Abbreviation: PMF, proton motive force.