INTRODUCTION
Ischemic injury to developing white matter (WM) is of considerable clinical interest. Although the immature central nervous system (CNS) is generally regarded to be more resistant than the adult CNS to such insults, it is now known that an ischemic episode between 23 and 32 weeks gestation can result in a remarkably selective pattern of injury to the periventricular WM (1–3). In the United States, approximately 57,000 low birthweight infants (<1500 g) are born each year and while recent advances in neonatal medicine mean that around 90% of these patients survive, sadly 10% show signs of cerebral palsy of which injury to the periventricular WM is the most common neuropathological correlate (4). In addition, recent estimates suggest that a cerebrovascular event occurs in around 1 in 4000 term births, although many more cases may go unrecognised, further illustrating the enormous problem ischemic injury to the developing brain poses to clinicians (5, 6).
This pattern of pathology was first noted by the great Russian academic Virchow in 1867 (7). A century later, it was termed periventricular leukomalacia (PVL) by Banker and Larrouche when describing "necrosis of the white matter dorsal and lateral to the external angles of the lateral ventricles" (8). PVL is now considered to consist of two main components: a focal site of injury characterised by necrosis of all cell types present and a diffuse pattern of injury which appears to affect only developing oligodendroglia leading to marked hypomyelination (9). It was traditionally thought that the lesion was entirely ischaemic in origin, although numerous reports now suggest an infective/inflammatory contribution (10,11). The vulnerability of the developing human brain to ischaemia has been replicated in numerous models including mid-to-late gestation sheep, 1 day old piglets and 5 to 7 day old rats (1,12–15). This has led to an intensive investigation of the physiology behind a pathology now acknowledged to be the leading cause of neurological disability in infants surviving neonatal intensive care (9,16). This review will focus on the effects of ischemia upon developing WM, exploring the reasons behind the sensitivity of the tissue and the experimental data on the response of the constituent cell types to energy deprivation.
WHY IS IMMATURE WHITE MATTER SO VULNERABLE TO ISCHEMIA?
Cessation of blood flow to the brain results in a rapid drop in ATP levels and consequently a loss in ionic homeostasis. The majority of the energy consumed by the CNS is used to power the Na+-K+ ATPase, which maintains a high concentration of Na+ outside the cell and a relatively high K+ concentration within the cell (17). The concentration gradients of these two ions are then utilised by a range of transporters to maintain the concentration gradients of other ions, for example, Ca2+ via the Na+-Ca2+ exchange protein. In the event of energy deprivation, the Na+-K+ ATPase fails leading to a rise in extracellular K + concentration, membrane depolarisation and the opening or reversal of numerous voltage sensitive ion channels or electrogenic transporters respectively. But why should the brain of the pre-term infant be deprived of energy?
VASCULAR ANATOMY AND PHYSIOLOGY OF CEREBRAL WHITE MATTER
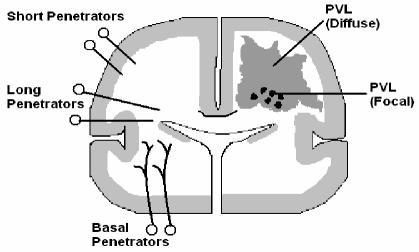
The vascular biology of developing periventricular WM appears to contribute to the predisposition of the area to ischemic injury for several reasons: blood to the periventricular WM is supplied by long and short penetrating arteries, which branch from the middle cerebral artery (18). Even towards the end of gestation this vasculature is not fully developed; both the long and short penetrating arteries are relatively few in number with few branches (18). Thus the arterial end zones are quite some distance from the periventricular region, which may lead to severe ischemia should cerebral blood flow decline.
To compound this issue, cerebral WM receives a relatively low blood flow during development: at 1.6 to 3 mL 100g-1 min-1 just 25% of that of cortical grey matter and less than half of the accepted value for cell viability in the adult brain (19–22). Such a low level of flow means that the margin by which blood flow can fall before injury occurs is very small. A limited vasodilatory capacity, i.e. the ability of the vasculature to dilate or constrict depending upon changes in blood pressure, has also been reported in premature infants (9). One study reported a four fold increase in the risk of PVL following identification of a "pressure-passive" cerebral circulation further illustrating the potential for vascular disturbances to cause serious injury in this region (23).
OLIGODENDROGLIAL INJURY
The most common neuropathological feature of ischemic injury to the periventricular WM is the diffuse injury of the oligodendroglial population and subsequent hypomyelination (24, 25). By defining the oligodendrocyte lineage in terms of the surface antigens expressed, the populating oligodendroglia present during the time of peak vulnerability in human cerebral WM have been identified as late oligodendrocyte progenitors or oligodendrocyte precursor cells (OPCs) (26). Many studies before and since have been able to demonstrate a remarkable sensitivity of these actively differentiating cells to ischaemic and oxidative stresses, resulting in the description of maturation-dependant characteristics which may be of crucial importance in the pathogenesis of ischemic injury (1,27–29).
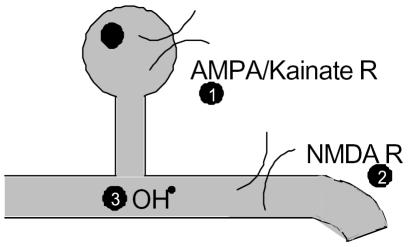
Glutamate, the most abundant neurotransmitter in the brain, has been shown to play an important role in the pathophysiology of OPC death. During ischaemia, extracellular glutamate levels rise dramatically and elevated glutamate levels have been observed in both animal models and clinically (30,31). When this happens both non-NMDA and NMDA receptors are over-activated (excitotoxicity), leading to a toxic Ca2+ flux in to the cell causing OPC damage and death (28–33). NMDA receptors have only recently been demonstrated in both neonatal and mature oligodendrocytes (32–35), while the expression of non-NMDA receptors in OPCs has been known for some time and shown to coincide with the window of vulnerability to ischaemia (12, 28, 36). Intriguingly, both the NMDA and non-NMDA receptors expressed in developing WM display curious alterations in their physiology which may contribute to the intrinsic susceptibility of the tissue. Oligodendrocyte NMDA receptors show only a weak voltage dependant block by Mg2+ compared with the classically described neuronal receptor. This means that these receptors permit a greater Ca2+ current than one might expect under the conditions of glutamate release and membrane depolarisation experienced during ischemia (32). In addition, AMPA/Kainate receptors expressed in developing oligodendroglia show reduced expression of the GluR2 subunit and so exhibit an increased permeability to Ca2+ (37–40). Remarkably, expression of this subunit has even been shown to be down-regulated following oxygen-glucose deprivation (OGD) preconditioning in an in vitro model (28).
While the protective effects of specific antagonists to glutamate receptors has been confirmed in vitro, situ and vivo (29, 32–34), the use of such drugs clinically is currently prevented by intolerable side-effects. These include renal toxicity due to poor water solubility in the case of AMPA receptor antagonists and behavioural effects due interference with synaptic function for NMDA receptor antagonists (41–43). Recent work, however, has revealed that these receptors may yet prove to be viable therapeutic targets as the use of the anti-convulsant topiramate has been shown to attenuate selective WM damage in a rodent model of WM injury (12). Xenon, a non-toxic anaesthetic gas that reduces neurotransmitter release and antagonizes NMDA receptors, has also been shown to provide significant neuro-protection in neonatal rats (44). In addition, the unusual subunit composition of oligodendrocyte NMDA receptors offers the potential of a novel drug target away from synaptic receptors (32, 33). In a recent article on this subject it was noted that these receptors contain the NR3 subunit, which is responsible for the decreased Mg2+ block seen in WM NMDA receptors (45). Currently, there are no selective agonists or antagonists for this subunit and Lipton suggests the development of such drugs may be of great importance for future clinical interventions (45).
There are many potential sources for glutamate release during ischemia. Release from axons is one possibility and glutamate release/depletion during ischaemia has been reported in optic nerve and spinal cord models (35, 46). This liberation could potentially occur via the reversal of electrogenic Na+-dependant glutamate transporters due to the conditions of ischemia. For example, membrane depolarisation and a concurrent increase in intracellular Na+, due to an inactivating Na+ conductance, was suggested in the spinal cord study (46) while release following axonal disruption is another possibility. The release of glutamate from astrocytes, following cell death and clasmatodendrosis (loss of cell processes), has also been suggested (47). In a companion paper, prevention of Na+-K+-Cl− co-transporter (NKCC) mediated astrocyte swelling neatly prevented non-NMDA receptor mediated rises in intracellular Ca2+ in neighbouring oligodendroglia during OGD, indicating a potential role for swelling mediated glutamate release (35). As well as receptor-mediated glutamate cell death, a non-receptor mediated affect may also operate in oligodendrocytes. Activation of a glutamate-cystine exchanger can lead to a reduction in cellular levels of cystine and hence decreased synthesis of the important antioxidant glutathione (48). This leaves the cell vulnerable to free radical attack, another weakness of OPCs.
That ischemia and reperfusion lead to the generation of free radicals is well established and OPCs have been demonstrated to be highly vulnerable to these species compared to mature oligodendrocytes (27,48,49). Cystine deprivation has been used to demonstrate the sensitivity of OPCs to oxidative stress caused by ensuing glutathione exhaustion, a treatment which has no effect upon the viability of mature oligodendrocytes (27,50). The exact nature of the discrepancy between the two developmental stages still requires further investigation but may be the result of a developmental delay in the maturity of anti-oxidant defences such as glutathione peroxidase and/or catalase (9). Volpe suggests that, should these defences be overwhelmed, hydrogen peroxide accumulates and combines with Fe2+ accumulated during differentiation. This may then lead, via the Fenton reaction, to the production of hydroxyl ions and cell death (9). Although devastating in effect this mode of injury is potentially the most treatable via clinically safe anti-oxidants such as vitamin E, which have the ability to rescue OPCs from free radical mediated death (Volpe unpublished data). Encouragingly, the phase III Stroke-Acute Ischemic NXY treatment trial (SAINT 1) provided evidence that NXY-059, a free-radical-trapping agent that has been neuroprotective in animal models, may be an effective adjunct to tissue plasminogen activator in the acute setting (51).
ASTROCYTES AND ISCHEMIA
Astrocytes are the most numerous cell type of the central nervous system but there is little information on their mechanism(s) of ischemic injury. Potential reasons for this include their relatively high resistance to energy deprivation and the difficulty of studying cell viability in vivo (52,53). Thus, most studies describing astrocyte pathophysiology have used cell culture methods to do so and significant differences, such as an attenuated rise in intracellular Ca2+ concentration in vitro when compared to in situ preparations, have been noted (52–54). Pooled studies on the response of astrocytes to ischemia have generated several potential causes of injury such as Ca2+ influx through L-type Ca2+ channels and Na+-Ca2+ exchange reversal, release from internal stores and cell swelling (47,52,54–57). Importantly, rapid astrocyte injury has been reported after hypoxia-ischemia in the neonatal striatum justifying investigations into astrocyte death in the immature brain (58).
With regard to the developing brain only two of the above studies attempted to use astrocytes in an immature in situ environment. The first of these used the rat optic nerve (RON) from animals aged between post-natal day 0–2, time points prior to the migration of oligodendroglia into the tract (52). The author was therefore able to study the response of in situ astrocytes to ischaemia by loading the Ca2+ dye FURA-2 into the whole optic nerve. The key findings of the paper were a toxic Ca2+ influx mediated by T- and L-type Ca2+ channels and a protective role for the Na+-Ca2+ exchanger. However, the P0–2 RON is more immature than the WM affected in human patients (1, 26). When similar experiments were performed using the P8–12 RON, in which myelination is just underway (a feature of the perinatal WM injured by ischemia), a different mechanism of injury was found (47). In this paper removal of extracellular Ca2+ was not protective (in fact it increased cell death), while removal of Na2+ Cl− or block of the NKCC with bumetanide was, suggesting cell swelling may be an important factor in cell death. Interestingly, analysis of volume changes during ischaemia did not reveal any changes although in experiments repeated in the absence of Ca 2+, significant swelling was observed. The authors concluded that ischaemia poses an osmo-regulatory challenge meditated by the NKCC and controlled by Ca2+-dependant mechanisms but this does not prevent cell death; the actual cause of cell death was not resolved (53). Future studies utilising recent advances in molecular biology may prove more successful in this respect. For example, the availability of transgenic mice generated by the laboratory of Dr. V Gallo at the Children's National Medical Centre, Washington DC, in which green fluorescent protein expression is under the control of the astrocyte specific GFAP promoter, should provide investigators with the means of assessing astrocyte injury more thoroughly in an in situ environment.
Astrocytes have long been ascribed a housekeeping function and ischaemia-induced alterations of the many functions they perform are capable of influencing the outcome of surrounding cells. For example, numerous studies of energy deprivation in the perinatal brain have reported astrocytic activation (59–62). Astrocytes may become "activated" by inflammatory mediators in a range of circumstances such as ischaemia and trauma leading to a characteristic up-regulation of GFAP, cellular hypertrophy, astrocyte proliferation and process extension (63). The consequences of astrocytic activation are not well understood and may be both beneficial and detrimental. For example, free radicals and inflammatory mediators such as TNFa may be released with the potential to cause injury while trophic factors such as TGFd and brain derived neurotrophic factor are also released and, remarkably, free radical scavenging is possible (64–69). In addition, astrocytes may affect the outcome of neighbouring cells through impaired K+ and glutamate uptake (70,71), further illustrating the potential importance of astrocyte protection.
AXON DAMAGE DURING ISCHEMIA
Banker and Larroche's (1962) description of PVL noted the presence of "retraction balls and clubs", that is, the presence of swollen axon cylinders and such injury will undoubtedly contribute to the phenotype of the ensuing disorder. Recently amyloid precursor protein, a marker of the integrity of fast axonal transport and therefore axon injury, has been detected in the brains of infants with WM injury (59, 72–74). Furthermore, in a developmental study on axon conduction during acute energy deprivation, a rapid decrease in the ability of RONs to recover electrical activity following OGD was noted around the onset of myelination (75).
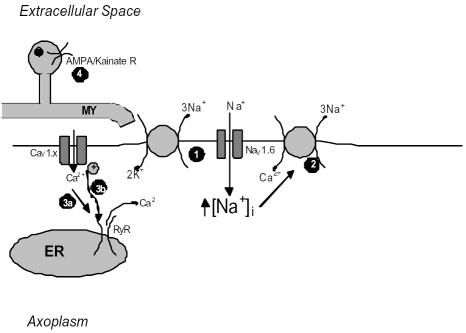
Although papers on the mechanisms of injury to myelinating axons have not been forthcoming, the response of myelinated central axons to energy deprivation has been extensively studied, primarily using the hypoxic rat optic nerve model (76–78). Initial investigations revealed that a toxic Ca2+ influx was mediated via reversal of the Na+-Ca2+ exchange protein following a rise in intra-axonal Na+ concentration (77). This Na+ loading, caused by non-inactivating Na+ channels, coupled with anoxia induced membrane depolarisation drives the electrogenic Na+-Ca2+ exchange protein into reverse operation with catastrophic consequences for axon function (79). Some later studies also found a protective role for specific voltage gated Ca2+ channel antagonists (78, 80).
Following on from these initial studies, investigators are now beginning to look at the mechanisms behind the injury sustained following both oxygen and glucose withdrawal; perhaps a more clinically relevant model given that the cessation of blood flow that occurs during stroke will decrease the availability of both oxygen and glucose. Using an acute brain slice preparation Tekkök and Goldberg reported AMPA/Kainate receptor mediated oligodendrocyte death in line with previous studies, but also used stimulus-evoked compound action potentials and immunohistochemistry to assess axon integrity (81). While Ca2+ removal preserved axon function following an insult, block of the Na+-Ca2+ exchange, in contrast to anoxia studies, provided only partial protection. Far more effective in preserving both compound action potential (CAP) conduction and neurofilament immunofluorescence was AMPA/Kainate blockade, a result which was not attributable to the protection of neuronal somata. The authors speculated that the underlying reasons for this might include myelin damage, an increase in tissue energy use or the loss of trophic support (81). Similar protection of optic nerve axons following OGD withdrawal has been reported in abstract form raising the possibility that in the event of a more severe metabolic insult, excitotoxicity may play an important role in acute axonal injury (82, 83).
Simulated ischemia has also led to reports of the "Trojan horse" of intra-axonal Ca2+ release" (84). Ouardouz et al. demonstrated that in rat dorsal columns, removal of bath Ca2+ did not improve post-ischemic CAP recovery and Ca2+ imaging experiments revealed that a rise in intracellular Ca2+ still persisted in such experiments (85). Ryanodine or blockers of the L-type Ca2+ channel voltage sensor (such as diltiazem) were protective during zero Ca2+ experiments. Subsequent immunoprecipitation and immunohistochemical studies revealed an association between L-type Ca2+ channels and ryanodine receptors on axons. These data led the authors to describe a mechanism similar to the excitation-contraction coupling seen in skeletal muscle where ischemic depolarisation sensed by L-type VGCCs activates ryanodine receptors on the endoplasmic reticulum (ER) leading to the release of damaging amounts of Ca2+. Such evidence indicates that ischemia-induced axon injury in the developing brain is likely to be the result of numerous pathways operating together leading to a catastrophic loss of function.
CONCLUSION
Although the developing brain is relatively resistant to ischemia, severe insults can result in a characteristically cortical-sparring pathology associated with both mental and physical disability. Numerous models for studying the effects of energy deprivation upon the developing brain are now available and have resulted in an ever growing body of literature on the subject. An understanding of the Byzantine pathophysiology of ischemia-induced white matter injury is steadily leading researchers towards the development of effective therapeutic interventions in the near future.
Figure 1.
Coronal section of cerebrum. The focal (black circles) and diffuse (grey shading) components of PVL are shown in one hemisphere and the cerebral vascular supply in the other. The long and short penetrating arteries supply the cerebral WM, as shown. From (9).
Figure 2.
Oligodendrocyte injury during ischemia. Rising levels of glutamate over stimulate both NMDA and non-NMDA receptors leading to a toxic influx of Ca (1 & 2). In addition, a developmental lag in anti-oxidant defences can lead to the build up of lethal free radicals such as the hydroxyl radical (3).
Figure 3.
Diagram illustrating mechanisms of ischemic injury to CNS axons. Following failure of the Na+-K+ pump Na+ accumulates in the axoplasm via a non-inactivating Na+ conductance (1). This increase results in reversal of the membrane Na+-Ca2+ exchanger (2). Ca2+ influx mediated by voltage gated Ca2+ channels may activate ER ryanodine receptors through either Ca2+-induced or depolarisation coupled means (3a & b). Finally, over activation of AMPA/Kainate receptors on oligodendroglia (4) might also contribute to the loss of functional integrity of the axon.
REFERENCES
- 1.Back SA, Han BH, Luo NL, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22(2):455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy TE, Kohle SJ, Vannucci RC. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem. 1940;24(2):271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- 3.Kabat H. The greater resistance of very young animals to arrest of the brain circulation. Am J Physiol. 1940;130:588–599. [Google Scholar]
- 4.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50(5):553–62. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109(1):116–23. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13(6):499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Virchow R. Zur pathologischen Anatomie des Gehirns I. Congenitale Encephalitis und Myelitis. (In German) Virchows Arch Pathol Anat. 1867;38:129–142. [Google Scholar]
- 8.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 9.Volpe, J.J., Neurology of the Newborn. 2001: WB Saunders Company.
- 10.Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46(5):566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284(11):1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 12.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20(24):9235–41. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rees S, Stringer M, Just Y, Hooper SB, Harding R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Brain Res Dev Brain Res. 1997;103(2):103–18. doi: 10.1016/s0165-3806(97)81787-7. [DOI] [PubMed] [Google Scholar]
- 14.Vannucci RC, Connor JR, Mauger DT, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55(2):158–63. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Yue X, Mehmet H, Penrice J, et al. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol Appl Neurobiol. 1997;23(1):16–25. [PubMed] [Google Scholar]
- 16.Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28(6):405–14. doi: 10.1053/j.semperi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Astrup J, Sorensen PM, Sorensen HR. Oxygen and glucose consumption related to Na+-K+ transport in canine brain. Stroke. 1981;12(6):726–30. doi: 10.1161/01.str.12.6.726. [DOI] [PubMed] [Google Scholar]
- 18.Rorke LB. Anatomical features of the developing brain implicated in pathogenesis of hypoxic-ischemic injury. Brain Pathol. 1992;2(3):211–21. doi: 10.1111/j.1750-3639.1992.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 19.Altman DI, Powers WJ, Perlman JM, Herscovitch P, Volpe SL, Volpe JJ. Cerebral blood flow requirement for brain viability in newborn infants is lower than in adults. Ann Neurol. 1988;24(2):218–26. doi: 10.1002/ana.410240208. [DOI] [PubMed] [Google Scholar]
- 20.Greisen G, Borch K. White matter injury in the preterm neonate: the role of perfusion. Dev Neurosci. 2001;23(3):209–12. doi: 10.1159/000046145. [DOI] [PubMed] [Google Scholar]
- 21.Borch K, Greisen G. Blood flow distribution in the normal human preterm brain. Pediatr Res. 1998;43(1):28–33. doi: 10.1203/00006450-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Powers WJ, Grubb RL, Jr, Darriet D, Raichle ME. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5(4):600–8. doi: 10.1038/jcbfm.1985.89. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji M, Saul JP, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–32. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 24.Iida K, Takashima S, Ueda K. Immunohistochemical study of myelination and oligodendrocyte in infants with periventricular leukomalacia. Pediatr Neurol. 1995;13(4):296–304. doi: 10.1016/0887-8994(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 25.Paneth N, Rudelli R, Monte W, et al. White matter necrosis in very low birth weight infants: neuropathologic and ultrasonographic findings in infants surviving six days or longer. J Pediatr. 1990;116(6):975–84. doi: 10.1016/s0022-3476(05)80664-x. [DOI] [PubMed] [Google Scholar]
- 26.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18(16):6241–53. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA. 2003;100(11):6801–6. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fern R, Moller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci. 2000;20(1):34–42. doi: 10.1523/JNEUROSCI.20-01-00034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagberg H. Hypoxic-ischemic damage in the neonatal brain: excitatory amino acids. Dev Pharmacol Ther. 1992;18(3–4):139–44. [PubMed] [Google Scholar]
- 31.Silverstein FS, Naik B, Simpson J. Hypoxia-ischemia stimulates hippocampal glutamate efflux in perinatal rat brain: an in vivo microdialysis study. Pediatr Res. 1991;30(6):587–90. doi: 10.1203/00006450-199112000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 34.Micu I, Jiang Q, Coderre E, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2005;439(7079):988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 35.Wilke S, Thomas R, Allcock N, Fern R. Mechanism of acute ischemic injury of oligodendroglia in early myelinating white matter: the importance of astrocyte injury and glutamate release. J Neuropathol Exp Neurol. 2004;63(8):872–81. doi: 10.1093/jnen/63.8.872. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg PA, Dai W, Gan XD, et al. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71(2):237–45. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- 37.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8(1):189–98. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 38.Durand GM, Zukin RS. Developmental regulation of mRNAs encoding rat brain kainate/AMPA receptors: a northern analysis study. J Neurochem. 1993;61(6):2239–46. doi: 10.1111/j.1471-4159.1993.tb07465.x. [DOI] [PubMed] [Google Scholar]
- 39.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini-Giampietro DE, Bennett MV, Zukin RS. Are Ca(2+)-permeable kainate/AMPA receptors more abundant in immature brain? Neurosci Lett. 1992;144(1–2):65–9. doi: 10.1016/0304-3940(92)90717-l. [DOI] [PubMed] [Google Scholar]
- 41.Akins PT, Atkinson RP. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr Med Res Opin. 2002;18 (Suppl 2):s9–13. doi: 10.1185/030079902125000660. [DOI] [PubMed] [Google Scholar]
- 42.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 43.Low SJ, Roland CL. Review of NMDA antagonist-induced neurotoxicity and implications for clinical development. Int J Clin Pharmacol Ther. 2004;42(1):1–14. doi: 10.5414/cpp42001. [DOI] [PubMed] [Google Scholar]
- 44.Dingley, J., J. Tooley, H. Porter, and M. Thoresen. Xenon Provides Short-Term Neuroprotection in Neonatal Rats When Administered After Hypoxia-Ischemia. Stroke, 2005. [DOI] [PubMed]
- 45.Lipton SA. NMDA receptors, glial cells, and clinical medicine. Neuron. 2006;50(1):9–11. doi: 10.1016/j.neuron.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Stys PK. Na(+)-K(+)-ATPase inhibition and depolarization induce glutamate release via reverse Na(+)-dependent transport in spinal cord white matter. Neuroscience. 2001;107(4):675–83. doi: 10.1016/s0306-4522(01)00385-2. [DOI] [PubMed] [Google Scholar]
- 47.Thomas R, Salter MG, Wilke S, et al. Acute ischemic injury of astrocytes is mediated by Na-K-Cl cotransport and not Ca2+ influx at a key point in white matter development. J Neuropathol Exp Neurol. 2004;63(8):856–71. doi: 10.1093/jnen/63.8.856. [DOI] [PubMed] [Google Scholar]
- 48.Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci. 1993;13(4):1441–53. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41(5):599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Yonezawa MM, Back SA, Gan X, Rosenberg PA, Volpe JJ. Cystine deprivation induces oligodendroglial death: rescue by free radical scavengers and by a diffusible glial factor. J Neurochem. 1996;67(2):566–73. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
- 51.Lees KR, Zivin JA, Ashwood T, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354(6):588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 52.Fern R. Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ. J Neurosci. 1998;18(18):7232–43. doi: 10.1523/JNEUROSCI.18-18-07232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13(8):3510–24. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duffy S, MacVicar BA. In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes. J Neurosci. 1996;16(1):71–81. doi: 10.1523/JNEUROSCI.16-01-00071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bondarenko A, Svichar N, Chesler M. Role of Na+-H+ and Na+-Ca2+ exchange in hypoxia-related acute astrocyte death. Glia. 2005;49(1):143–52. doi: 10.1002/glia.20107. [DOI] [PubMed] [Google Scholar]
- 56.Holgado A, Beauge L. The Na(+)-Ca2+ exchange system in rat glial cells in culture: activation by external monovalent cations. Glia. 1995;14(2):77–86. doi: 10.1002/glia.440140202. [DOI] [PubMed] [Google Scholar]
- 57.MacVicar BA. Voltage-dependent calcium channels in glial cells. Science. 1984;226(4680):1345–7. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- 58.Martin LJ, Brambrink AM, Lehmann C, et al. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42(3):335–48. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 59.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16(4):296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 60.Farkas E, Institoris A, Domoki F, Mihaly A, Luiten PG, Bari F. Diazoxide and dimethyl sulphoxide prevent cerebral hypoperfusion-related learning dysfunction and brain damage after carotid artery occlusion. Brain Res. 2004;1008(2):252–60. doi: 10.1016/j.brainres.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62(5):441–50. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 62.Sizonenko SV, Kiss JZ, Inder T, Gluckman PD, Williams CE. Distinctive neuropathologic alterations in the deep layers of the parietal cortex after moderate ischemic-hypoxic injury in the P3 immature rat brain. Pediatr Res. 2005;57(6):865–72. doi: 10.1203/01.PDR.0000157673.36848.67. [DOI] [PubMed] [Google Scholar]
- 63.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–7. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 64.Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F. Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta. J Neurosci. 1998;18(23):9594–600. doi: 10.1523/JNEUROSCI.18-23-09594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhandapani KM, Hadman M, De Sevilla L, Wade MF, Mahesh VB, Brann DW. Astrocyte protection of neurons: role of transforming growth factor-beta signaling via a c-Jun-AP-1 protective pathway. J Biol Chem. 2003;278(44):43329–39. doi: 10.1074/jbc.M305835200. [DOI] [PubMed] [Google Scholar]
- 66.D'Souza SD, Alinauskas KA, Antel JP. Ciliary neurotrophic factor selectively protects human oligodendrocytes from tumor necrosis factor-mediated injury. J Neurosci Res. 1996;43(3):289–98. doi: 10.1002/(SICI)1097-4547(19960201)43:3<289::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 67.Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem. 1999;72(6):2334–44. doi: 10.1046/j.1471-4159.1999.0722334.x. [DOI] [PubMed] [Google Scholar]
- 68.Lucius R, Sievers J. Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes. Brain Res. 1996;743(1–2):56–62. doi: 10.1016/s0006-8993(96)01029-3. [DOI] [PubMed] [Google Scholar]
- 69.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50(4):307–20. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 70.Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53(2):212–24. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- 71.Ransom BR, Orkand RK. Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci. 1996;19(8):352–8. doi: 10.1016/0166-2236(96)10045-x. [DOI] [PubMed] [Google Scholar]
- 72.Arai Y, Deguchi K, Mizuguchi M, Takashima S. Expression of beta-amyloid precursor protein in axons of periventricular leukomalacia brains. Pediatr Neurol. 1995;13(2):161–3. doi: 10.1016/0887-8994(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 73.Meng SZ, Arai Y, Deguchi K, Takashima S. Early detection of axonal and neuronal lesions in prenatal-onset periventricular leukomalacia. Brain Dev. 1997;19(7):480–4. doi: 10.1016/s0387-7604(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 74.Ohyu J, Marumo G, Ozawa H, et al. Early axonal and glial pathology in fetal sheep brains with leukomalacia induced by repeated umbilical cord occlusion. Brain Dev. 1999;21(4):248–52. doi: 10.1016/s0387-7604(99)00018-2. [DOI] [PubMed] [Google Scholar]
- 75.Fern R, Davis P, Waxman SG, Ransom BR. Axon conduction and survival in CNS white matter during energy deprivation: a developmental study. J Neurophysiol. 1998;79(1):95–105. doi: 10.1152/jn.1998.79.1.95. [DOI] [PubMed] [Google Scholar]
- 76.Stys PK, Ransom BR, Waxman SG, Davis PK. Role of extracellular calcium in anoxic injury of mammalian central white matter. Proc Natl Acad Sci USA. 1990;87(11):4212–6. doi: 10.1073/pnas.87.11.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992;12(2):430–9. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J Neurophysiol. 1995;74(1):369–77. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- 79.Leppanen L, Stys PK. Ion transport and membrane potential in CNS myelinated axons. II. Effects of metabolic inhibition. J Neurophysiol. 1997;78(4):2095–107. doi: 10.1152/jn.1997.78.4.2095. [DOI] [PubMed] [Google Scholar]
- 80.Brown AM, Westenbroek RE, Catterall WA, Ransom BR. Axonal L-type Ca2+ channels and anoxic injury in rat CNS white matter. J Neurophysiol. 2001;85(2):900–11. doi: 10.1152/jn.2001.85.2.900. [DOI] [PubMed] [Google Scholar]
- 81.Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21(12):4237–48. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tekkok SB, B.E.a.R.B. Older adult animals and the mechanisms of ischemic white matter injury. Program No. 100.12. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2005.
- 83.B.R. Ransom, S.B.T. Perceptor pharmacology of excitotoxic injury in mouse optic nerve. in Program No. 100.13. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2005. 2005.
- 84.Ransom BR, Brown AM. Intracellular Ca2+ release and ischemic axon injury: the Trojan horse is back. Neuron. 2003;40(1):2–4. doi: 10.1016/s0896-6273(03)00602-0. [DOI] [PubMed] [Google Scholar]
- 85.Ouardouz M, Nikolaeva MA, Coderre E, et al. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40(1):53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]