Abstract
Cardiac patients often have sinus arrhythmia of non-respiratory origin [erratic sinus rhythm” (ESR)]. ESR was quantified using hourly Poincaré and power spectral HRV plots from normal-to-normal interbeat intervals (N-Ns) and hourly values of the short-term fractal scaling exponent and correlations of N-Ns in N=60 non-survivors and N=66 randomly-selected survivors in the Cardiac Arrhythmia Suppression Trial. Hours were coded (ABN) as normal (0), borderline (0.5) or ESR (1). T-tests compared ABN for N=2413 paired hours at baseline and on therapy. ABN was higher in non-survivors (0.38 ± 0.44 vs. 0.28 ± 0.40, baseline and 0.51 ± 0.45 vs. 0.34 ± 0.43, on therapy, p<0.001). Increased ABN with treatment was greater in non-survivors. Normal hours at baseline (RR=0.77, 095% CI=0.62−0.96, p=0.018) and on treatment (RR=0.47, 95%CI=0.39−0.58) were significantly associated with decreased mortality compared to ESR. Quantification of ESR may identify more vulnerable patients or help monitor the effects of pharmacological treatment.
Keywords: heart rate, risk factor, mortality, post-MI, anti-arrhythmic
The analysis of heart rate variability (HRV) provides information about cardiac autonomic function. However, we have found that many older subjects and patients with cardiovascular disease have an increased beat-to-beat variability that is irregular and does not reflect underlying respiratory sinus arrhythmia (1). We have called this erratic rhythm. The presence of erratic rhythm, which is frequently episodic, increases values for short-term HRV indices such as rMSSD [root mean square of successive differences of normal-to-normal (N-N) interbeat intervals in ms], pNN50 [the % of N-N intervals >50 ms different from the previous interval) and high frequency power (the amount of variance in N-N intervals at respiratory frequencies, i.e., 0.15−0.4 Hz) that quantify beat-to-beat variability. Thus, in the presence of erratic rhythm, increased short-term HRV does not, as it does for younger people, necessarily reflect greater parasympathetic control of heart rate. A high prevalence of erratic rhythm may also explain why HRV measures that are believed to reflect parasympathetic function have relatively little predictive value for outcomes. At the same time, some “non-linear” HRV measures like the short-term fractal scaling exponent, (DFA1), and (SD12), the ratio of the axes of an ellipse fitted to the Poincaré plot do capture the presence of erratic rhythm (2,3). DFA1 is reduced and SD12 is increased when there is a more irregular heart rate pattern. Because erratic rhythm tends to be episodic, non-linear HRV calculated on an hourly basis should be more sensitive to the presence of erratic rhythm than 24-hour measures. In addition, erratic rhythm can be seen visually as a relatively disorganized power spectral or Poincaré plot.
We hypothesized that post-MI patients who have or develop an increased degree of erratic rhythm would be at higher risk of mortality than those who do not. We tested this hypothesis in the Cardiac Arrhythmia Suppression Trial (CAST). The CAST was conducted to determine if a reduction in ventricular premature beats in association with one of three randomized anti-arrhythmic therapies would improve survival in post-MI patients. Holter recordings were obtained at baseline and after the start of anti-arrhythmic therapy. To test our hypothesis, we used a semi-quantitative method to compare the degree of erratic rhythm in baseline and post-treatment recordings in CAST patients who died during follow up and a randomly-selected comparison group of those who survived.
Materials and Methods
Patient population
The goal of the CAST was to test the hypothesis that suppression of VPCs would decrease mortality after MI (4). Patients were randomly assigned to encainide, moricizine, or flecainide, with flecainide omitted in the subgroup with the lowest EF (LVEF<30%). Patients with significant suppression of VPCs with a particular agent were continued on that agent or placebo. More complete information concerning study design may be found in the primary endpoint reports (5,6,7). In April, 1989, the Data and Safety Monitoring Board of the CAST recommended that the encainide and flecainide arms of the study be discontinued because of increased mortality in those arms, and CAST II was begun. Patients in CAST II were selected to be at higher risk than those in CAST I.
Pre-treatment (qualifying) tapes from participants in CAST (N=830) who had their VPCs successfully suppressed on their first, randomly-assigned anti-arrhythmic treatment and were continued on that agent were selected for analysis at the Washington University School of Medicine HRV Laboratory. All of the subjects in this cohort were also classified as having usable suppression tapes.
Subjects in the current case-control study were selected from among those with analyzed recordings using the following criteria: all of the subjects (N=68) who died and had recordings at both baseline and suppression were included. In addition, control subjects were randomly selected from among survivors, in such a way that every 10th subject (by ID) was selected, unless that subject had died, in which case the next person was selected. As a result, 75 comparison patients were identified for the study. Because subjects were required to have usable data on both recordings, data were analyzed from 64 of 75 comparison patients and 61 of 68 non-survivors. Of them, N= 47 were randomized to encainide, N=30 to flecainide and N= 48 to moricizine.
Clinical and demographic data
Clinical and demographic data were provided by the CAST coordinating center. Characteristics of the CAST patients and procedures for data validation have been previously reported (6).
Analysis of Holter Recordings
Tapes were analyzed on a Marquette SXP Laser Holter scanner (Marquette Electronics, Milwaukee, WI, software version 5.8) by an experienced research Holter technician using standard Holter analysis procedures. Beatstream files, representing the time and classification of each QRS complex, were transferred to a Sun computer (Sun Microsystems, Palo Alto, CA), where careful HRV analysis were performed using previously reported and validated techniques (8,9). The longest and shortest true normal-to-normal (N-N) intervals were identified for each tape, and intervals outside of these limits, as well as all ectopic beats, were excluded from all calculations and plots. All intervals that resulted from blocked atrial premature beats were excluded.
Detection of Erratic Rhythm
The presence of erratic rhythm was identified using hourly power spectral plots of 2-min averaged HRV, hourly Poincaré plots and hourly measures of DFA1 and the correlation coefficient between N-Ns. Each hour of each recording was categorized as normal, borderline or erratic. Details are provided below. For an hour to be acceptable for analysis, 50% of the data had to consist of normal-to-normal interbeat intervals.
Non-Linear HRV Variables and Erratic Rhythm
HRV variables which reflect erratic rhythm and were examined included: the short-term fractal scaling exponent (DFA1) and the N-N interbeat correlation coefficient (CORR). Although SD12, the ratio of the axes of an ellipse fitted to the Poincaré plot, was also calculated, it did not provide additional information and is not considered here.
1) DFA1
Detrended fluctuation analysis (DFA) quantifies the fractal scaling properties of the short-term R-R interval time series. Higher values for DFA reflect a more correlated time series, while markedly decreased values reflect a highly random time series. The method is a modified root-mean square analysis of random walks, which quantifies the presence or absence of fractal correlation properties, and has been validated for time series (2,10). The root-mean-square fluctuation of integrated and detrended times series is measured at different observation windows and plotted against the size of the window on a log-log scale. The details of this method have been described elsewhere (2). Fractal scaling exponents were determined on an hourly basis, for short-term (4−11 beats, DFA1). Only normal-to-normal (N-N) intervals were used for this calculation.
2) Autocorrelation coefficient with a lag of one
(CORR). The Pearson's correlation between the time series of N-N intervals and the same time series offset by one beat was calculated for each hour. While not strictly speaking a non-linear HRV measure, Increased irregularity in the heart rate time series resulted in decreased values for the interbeat correlation coefficient.
Abnormal Poincaré Plots and Erratic Rhythm
Poincaré plots (i.e., plots of each N-N interval vs. the next) are a graphical representation of the organization of heart rate patterns. Normal-looking one-hour Poincaré plots of N-N interbeat intervals are ellipsoid or mildly comet-shaped, with few data points outside the ellipsoid part of the figure. The left hand side of Figure 1 (a-d) shows Poincaré plots categorized as normal. When the borderline plots in Figure 2 a and b are compared to the normal plots in figure 1, it is clear that the ellipsoid or comet-shaped core is less well defined and there are numerous data points outside of the core. The clearly erratic plots (Figures 2c-f) are readily identifiable because they are fan shaped and lack a single clearly defined ellipsoid or comet-like core.
Figure 1.
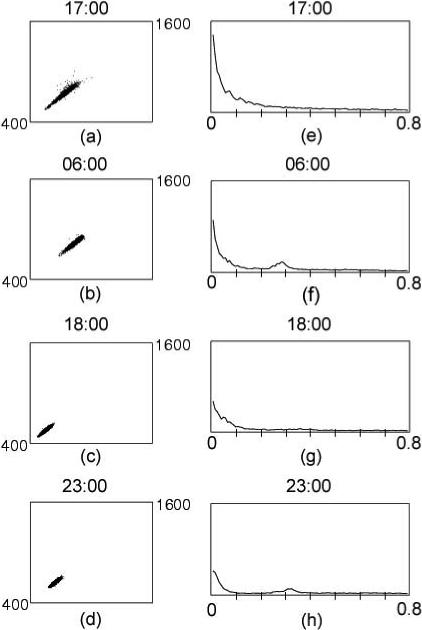
Examples of hourly Poincaré and FFT plots coded as normal from both the day and nighttime periods and in subjects with higher and lower HRV. The corresponding left and right hand plots are from the same patients. DFA1 ranged from 1.18 for Figure 1a to 0.87 for Figure 1b. CORR ranged from 0.96 for Figure 1a to 0.93 for Figure 1d.
Figure 2.
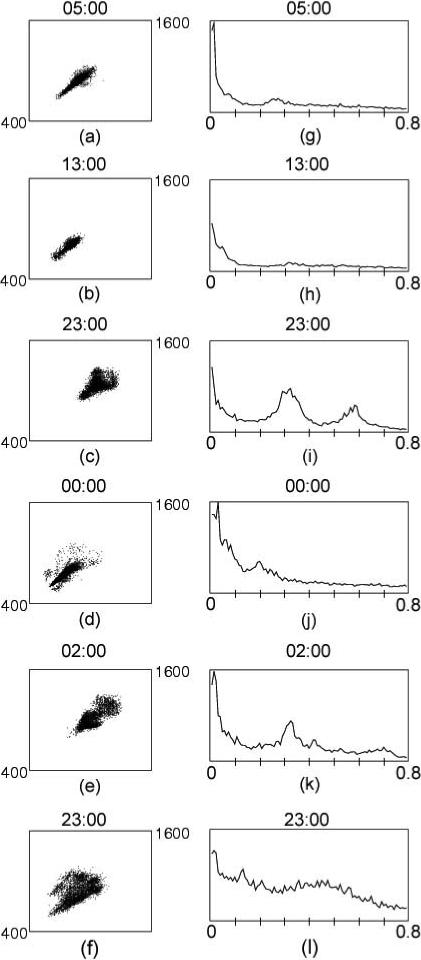
Examples of FFTs and Poincaré plots coded as borderline (2a, 2b) and erratic (2c-2f). Among the borderline plots, DFA1 was 0.77 in Fig. 2a and 0.86 in Fig. 2b, and CORR was 0.87 in Fig 2a and 0.86 in Fig. 2b. Among the erratic plots, DFA1 ranged from 0.58 in Fig. 2f to 0.93 in Fig. 2d. CORR ranged from 0.89 in Fig. 2c to 0.48 in Fig. 2f.
Abnormal Hourly Power Spectral Plots and Erratic Rhythm
Frequency domain HRV analysis partitions the variance of the heart rate (actually heart period) into its underlying frequency components using the fast Fourier transform (FFT). The amount of variance (power) at each underlying frequency can be displayed graphically. In each plot, the y-axis reflects the amplitude of the variance at each frequency and the x-axis is the underlying frequency in Hz. The Discrete Fast Fourier Transform method was used to perform power spectral analysis (9). The NN interval time series was sampled at 1024 samples every 5 min. Ectopic beats and artifact were splined over, but at least 80% NN intervals had to be present for a 5-min segment to be used. These graphs were made by plotting HRV for every 5 minutes and then averaging the plots for every hour. At least five of twelve 5 min segments had to be usable for a plot to be made, otherwise that hour was excluded.
Abnormal variation in heart rate patterns is visible as a more disorganized (broadband) FFT. The right hand side of Figure 1 (e-h) provides examples of normal-appearing hourly FFT plots, from both the day and nighttime periods, in subjects with high and low HRV and corresponds to the Poincaré plots seen on the left hand side. As can be seen from the figure, normal FFTs can be characterized as “organized-looking” with a distinct 1/f distribution of spectral power in the region between 10−2 and 10−4 Hz and relatively little power (area under the curve) above the high frequency band (0.4 Hz). Nighttime and naptime plots tend also to show a distinct peak in the high frequency band, associated with higher respiration-associated vagal modulation of heart rate during sleep (Figure 1f and h). This peak was generally absent during the daytime awake periods.
The right hand side of Figure 2 (g-l) provides examples of FFTs categorized as borderline or erratic. In contrast to those shown in Figure 1, these plots are irregular and disorganized and, in some, the distinct 1/f distribution of spectral power described above is not seen. Because of the abnormal rhythm, significant power is seen beyond the high frequency band (i.e., >0.4 Hz). These FFTs correspond to the Poincaré plots shown in the left hand side. Figures 2 a and 2b show FFTs categorized as borderline, and the remainder (Figures 2i-l) were erratic.
Categorization of Hourly HRV Using FFT and Poincaré Plot Characteristics in Conjunction with Non-Linear HRV Cutpoints
Each hour was then categorized as: 0=normal, 0.5=borderline, 1=erratic based on the coding of four characteristics (DFA1, CORR, the FFT plot, and the Poincaré plot). Qualifying hours had ≥50% N-Ns and qualifying patients had ≥8 useable hours during both pre-treatment and on-therapy recordings. Categorization was done as follows: first, each of the 4 characteristics was coded individually. For the FFT and Poincaré plots, the coding was normal, borderline or erratic based on the appearance of the plot as described above. Plots that could not be obviously categorized were considered borderline.
For the DFA1 and CORR, the coding was normal, potentially normal, potentially erratic and erratic. Based on our prior pilot studies using ROC curves, DFA1 DFA1>1 or CORR >0.9 was coded as definitely normal. DFA1 0.85−0.99 or CORR 0.85−0.89 was coded as potentially normal. DFA1 or CORR =0.81−0.84 was coded as potentially erratic. DFA1 or CORR<0.80 was coded as definitely erratic.
To create the single value for each hour, the following criteria were used. If the coding was consistent between all criteria (i.e., all normal, all borderline or all erratic) that category was assigned. If the plots were consistently normal (or consistently erratic) and the DFA1 and/or the CORR range was potentially normal (or potentially erratic), that hour was classified as normal (or erratic). Similarly, if the plots were consistently borderline, and the CORR or DFA1 was not clearly normal or erratic, these hours were categorized as borderline.
If the coding was consistent between 3 of the 4 criteria and the inconsistency was that one of the plots was borderline, the coding of the 3 consistent criteria was assigned. Totally inconsistent hours were coded as borderline. Often this occurred because the erratic rhythm began early in the period but stopped or, conversely, it began late in the period, resulting in near normal values for DFA1 or CORR but clear abnormalities in the plots. However, if DFA1 was <0.70, the combined code was always abnormal.
Statistical Analyses
Age, gender and selected clinical characteristics, including survival in days, were compared between survivors and non-survivors using t-tests for continuous variables and chi-square analyses for categorical variables. Paired t-tests compared baseline abnormality categories and changes in abnormality categories for each hour of the recordings between survivor and non-survivors. Binary logistic regression analysis assessed the relative risk of mortality for erratic hours on the first and second recording. Statistical significance was set at p<0.05. The software was SPSS 11.0 (SPSS, Chicago, IL).
Results
Patient Characteristics
There were 61 non-survivors and 64 surviving control subjects with sufficiently hourly data for both recordings. Non-survivors were similar in age (62.6±8.0 vs. 60.8±10.0 yrs), gender (48M, 13F vs. 56M, 8F) and NYHA class (1.5 vs. 1.3) to survivors, but had significantly lower LVEFs (32.0 ± 11.7 vs. 36.0 ± 11.7%). There was no significant different in beta blocker use (37.5% survivors, 29.5% non-survivors, p=0.26) but consistent with their lower LVEF's there was a trend to greater use of digoxin (21.9% survivors, 36.1 % non-survivors, p=0.06) in those who died.
Figure 3 shows the total number of normal (0), borderline (0.5) and erratic (1) hours at baseline and after antiarrhythmic treatment. There were 2651 paired hours on baseline and treatment recordings, with no difference in number of qualifying hours between survivors and non-survivors. Mean hourly abnormality score was higher in those who died, both at both baseline (0.40 ± 0.44 non-survivors vs. 0.27 ± 0.40 controls) and after anti-arrhythmic therapy (0.51 ± 0.45 in non-survivors vs. 0.34 ± 0.43 in controls), p<0.001. Moreover, although abnormality scores increased significantly in both groups, the change in abnormality score in association with anti-arrhythmic therapy was significantly greater among those who died (0.64 ± 0.42 in non-survivors vs. 0.13 ± 0.51 in survivors)
Figure 3.
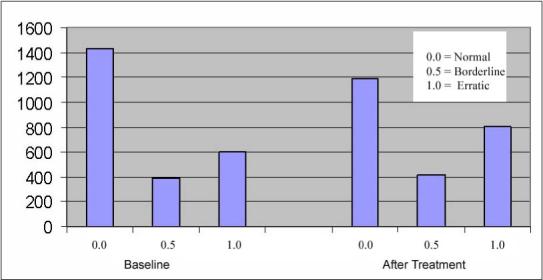
Bar graph comparing the total number of normal (0), borderline (0.5) and erratic (1) hours on the baseline recordings and on the post-antiarrhythmic treatment recordings in the entire study cohort.
Figure 4 shows the number of hours in each abnormality category for non-survivors and surviving controls for both recordings. By the design of this case-control study, if there was no difference in survival, the number hours for survivors and non-survivors should be approximately equal in each category. As the figure shows, the number of hours categorized as normal decreased in both groups, but the decrease was greater among non-survivors. The number of hours categorized as borderline was similar between groups and decreased in both groups. The number of hours categorized as erratic increased in both groups, but the increase was greater in non-survivors.
Figure 4.
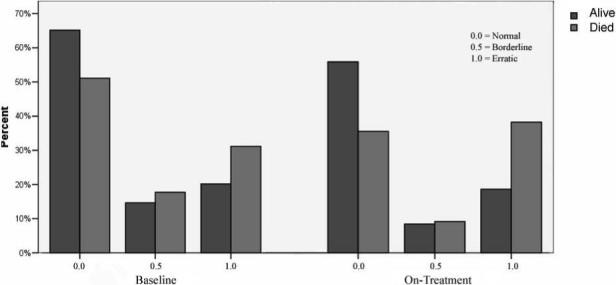
Bar graphs showing the relative percent of hours in each abnormality category for survivors vs. non-survivors for both the baseline and on-treatment recordings.
When survival was compared between categories of abnormality scores on the baseline recording, hours having normal heart rate patterns were significant associated with reduced risk of mortality compared with hours having erratic rhythm on the first recording (RR=0.77, 095% CI=0.62−0.96, p=0.018). This relationship was stronger for the on treatment recording where hours classified as normal (RR=0.47, 95%CI=0.39−0.58) or borderline (RR=0.67, 95% CI= 0.52−0.85, all p≤0.001) were associated with better survival.
Discussion
Our study confirms the potential clinical importance of erratic heart rate patterns among post-MI patients. Results confirmed the hypothesis that progression to a greater degree of erratic rhythm was associated with increased mortality. Moreover, erratic heart rate patterns at baseline were already associated with increased risk of mortality and the risk increased after anti-arrhythmic therapy. We found no survival difference between completely normal-appearing rhythms and those not obviously erratic (i.e., borderline), suggesting that both the presence and development of easily identifiable, clearly erratic rhythms, conveys higher risk. Because the CAST study lacked a placebo control group, it is not clear whether the increased degree of erratic rhythm was due to treatment or disease progression, but the fact that most of the recordings were 2 weeks apart supports the first explanation.
In an earlier study, we applied similar methodology to a subset of the Cardiovascular Health Study (CHS), an NIH-sponsored population-based study of cardiovascular and cerebrovascular disease in the elderly (1). Our purpose was to identify HRV measures that were exaggerated by erratic rhythm, appearing healthier when then were in fact more abnormal. We found that short-term HRV measures like rMSSD were markedly higher in those with a high degree of erratic rhythm. Consistent with our findings for the baseline Holters in CAST, a greater degree of erratic rhythm in the population-dwelling elderly was independently associated with increased mortality on 5-year follow up.
The exact nature of erratic rhythm is unknown. However, the idea that the sino-atrial node provides a single focus of origin for each beat has been superseded by the understanding that sinus rhythm is controlled by multiple dominant pacemaker sites integrated by a complex ganglionic network (11). It is possible therefore that erratic rhythm is a consequence of the breakdown of this system and might represent a pre-clinical form of sick sinus syndrome, although sick sinus syndrome per se is found in only 1 in 600 cardiac patients over 65 years old (12). Other evidence suggests that erratic rhythm can be a consequence of high sympathetic activation. In a study of normal volunteers, increasing doses of norepinephrine resulted in an increase in the degree of sinus arrhythmia of non-respiratory origin (13). This result is consistent with the finding that CHF patients with “complex” Poincaré plots (which we would have classified as clearly erratic) had a significantly higher serum norepinephrine level than patients who did not (14).
In our study, we used multiple criteria to identify erratic rhythms and quantified each hour of the recording based on these criteria, rather than making a single assessment of a 24-hour plot. Although the term “erratic rhythm” was not used, abnormal 24-hour Poincaré plots have identified higher-risk patients with CHF. Woo et al characterized Poincaré plots in patients with advance heart failure compared to normals (15). Seven of nine patients in the “complex” group died suddenly within 6 months of the recording. Brouwer et al examined the association between Poincaré plot patterns and outcome in patients with mild to moderate heart failure (16). Abnormal 24-hour Poincaré plots were the only HRV index that identified an increased risk of mortality. Similarly, Bonaduce et al found a significantly greater proportion of abnormal Poincaré plots among non-survivors compared to survivors in a CHF population (17).
Poincaré plot analysis has been applied post-MI as well. Huikuri et al used both 24-hour and hourly Poincaré plots to compare post-MI patients with either recorded ventricular tachycardia (VT) or VT inducible on EP study and post-MI patients who had neither with age- and gender-matched healthy controls (18). All of the VT patients had abnormal plots, which became more abnormal during the 1-hour period before the onset of VT. Complex patterns were seen in many VT patients, but in none of the other two groups. In another study from the same investigators, post-CABG patients demonstrated increased randomness of the heart period time series (i.e., more erratic rhythm), quantified as markedly increased values for twenty-four-hour SD12, compared to the same patients before surgery. Moreover, results suggested that a more random heart rate pattern predicted ischemic events in these post-CABG patients (19).
The effect of treatment on heart rate variability has been used as a measure of treatment efficacy. However, our results suggest that monitoring the effect of therapy on the detailed underlying structure of heart rate patterns is important as well and, as we found in the CAST, could be a useful way to identify those who are adversely impacted. CHF patients treated with digitalis, for example, have been reported to have increases in vagally-modulated HRV (20). However, in the small number of patients treated with digitalis who had baseline and on-treatment Holters at our site, treatment with digitalis was associated with an increase in the degree of erratic rhythm which would also elevate short-term HRV measures (unpublished data), suggesting that simply quantifying the treatment effect of HRV is not sufficient in this population. The CAST drugs had an adverse effect on survival and likely, from our results, on heart rate patterns.
Limitations
The CAST population was chosen because of a high prevalence of VPCs and increased risk of sudden death. Although the study was conducted before the era of modern therapy, we frequently observe periods of erratic rhythm on Holters in the modern era and therefore believe that this remains an important potential factor whose prognostic significance needs to be tested. Further study in a population receiving current therapy will determine if this risk factor is independent of other clinical factors including left ventricular dysfunction.
While our method of characterizing heart rate patterns had provided support for our hypothesis, it is labor-intensive and semi-quantitative at best. Determinations had some degree of subjectivity. However, most of this was in the classification of plots as normal vs. borderline. Clearly abnormal plots, which were the type associated with decreased survival, were easily and unambiguously identified using this method. Decreased values for the 24-hour averaged short-term fractal scaling exponent also capture increased randomness of heart rate patterns, but we believe that more efficient and precise methods for detecting and quantifying the presence and degree of episodes of erratic rhythm will provide even better risk markers. Furthermore, short-term HRV calculated from erratic-rhythm free periods might identify subjects at higher risk of mortality, because they truly have decreased vagal control of heart rate.
Acknowledgments
I would like to thank Richard Cohen for his help with the figures.
Grant support: Supported by NHLBI R0-3 HL53776
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes Higher Heart Rate Variability is Not Better Heart Rate Variability: Results of Graphical and Non-Linear Analyses. Journal of Cardiovascular Electrophysiology. 2005;16:1–6. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- 2.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 3.Laitio T, Mäkikallio TH, Huikuri HV, Kentala ES, Uotila P, Jalonen JR, Helenius H, Hartiala J, Yli-Mäyry S, Scheinin H. Relation of heart rate dynamic to the occurrence of myocardial ischemia after coronary artery bypass grafting. Am J Cardiol. 2002;89:1178–1181. doi: 10.1016/s0002-9149(02)02300-7. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AE, Bigger JT, Jr., Wyse DG, Romhilt DW, Reynolds-Haertle RA, Hallstrom AP. Events in the Cardiac Arrhythmia Suppression Trial (CAST): mortality in the entire population enrolled. J Am Coll Cardiol. 1991;18:14–19. doi: 10.1016/s0735-1097(10)80210-4. [DOI] [PubMed] [Google Scholar]
- 5.Hallstrom A, Pratt CM, Greene HL, Huther M, Gottlieb S, DeMaria A Young JB. Relations between heart failure, ejection fraction, arrhythmia suppression and mortality: analysis of the Cardiac Arrhythmia Suppression Trial. J Am Coll Cardiol. 1995;25:1250–57. doi: 10.1016/0735-1097(94)00553-3. [DOI] [PubMed] [Google Scholar]
- 6.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Green HL. Mortality and morbidity in patients receiving encainide, flecainide or placebo. N Engl J Med. 1991;324:781–88. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 7.The Cardiac Arrhythmia Suppression Trial II Investigators Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–33. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 8.Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans Biomed Eng. 1986;9:900–4. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- 9.Rottman JN, Steinman RC, Albrecht P, Bigger JT, Jr., Rolnitzky LM, Swartz PJ. Efficient estimation of the heart period power spectrum suitable for physiologic or pharmacologic studies. Am J Cardiol. 1990;66:1522–24. doi: 10.1016/0002-9149(90)90551-b. [DOI] [PubMed] [Google Scholar]
- 10.Iyengar N, Peng C-K, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol. 1996;271:R1078–1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- 11.Schuessler RB, Boineau JP, Bromberg BI. Origin of the sinus impulse J Cardiovasc Electrophysiol. 1996;7:263–74. doi: 10.1111/j.1540-8167.1996.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 12.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity promoting understanding of sick sinus syndrome. Contemporary Reviews in Cardiovascular Medicine. Circulation. 2007:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 13.Tulppo MP, Mäkikallio TH, Seppänen T, Airaksinen KEJ, Huikuri HV. Heart rate dynamics during accentuated sympathovagal interaction. Am J Physiol. 1998;274(Heart Circ Physiol43):H810–H816. doi: 10.1152/ajpheart.1998.274.3.H810. [DOI] [PubMed] [Google Scholar]
- 14.Woo MA, Stevenson WG, Moser DK, Middlekauff HR. Complex heart rate variability and serum norepinephrine levels in patients with advanced heart failure. J Am Coll Cardiol. 1994;23:565–9. doi: 10.1016/0735-1097(94)90737-4. [DOI] [PubMed] [Google Scholar]
- 15.Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. Am Heart J. 1992;123:704–710. doi: 10.1016/0002-8703(92)90510-3. [DOI] [PubMed] [Google Scholar]
- 16.Brouwer J, van Veldhuisen DJ, Man in T'Veld AJ, Haaksma J, Dijk WA, Visser KR, Boomsma F, Dunselman PHJM, Lie KI for the Dutch Ibopamine Multicenter Trial Study Group Prognostic value of heart rate variability during long-term follow-up in patients with mild to moderate heart failure. J Am Coll Cardiol. 1996;28:1183–9. doi: 10.1016/s0735-1097(96)00279-3. [DOI] [PubMed] [Google Scholar]
- 17.Bonaduce D, Petretta M, Fortunato M, Vicario MLE, Apicalle A, Ras MAE, Nicolai E, Volpe M. Independent and incremental prognostic value of heart rate variability in patients with chronic heart failure. Am Heart J. 1999;138:273–284. doi: 10.1016/s0002-8703(99)70112-2. [DOI] [PubMed] [Google Scholar]
- 18.Huikuri HV, Seppanen T, Koistinen MJ, Airaksinen J, Ikaheimo MJ, Castellanos A, Myerburg RJ. Abnormalities in beat-to-beat dynamics of heart rate before the spontaneous onset of life-threatening ventricular tachyarrhythmias in patients with prior myocardial infarction. Circulation. 1996;93:1836–44. doi: 10.1161/01.cir.93.10.1836. [DOI] [PubMed] [Google Scholar]
- 19.Laitio TT, Mäkikallio T, Huikuri H, Kentala ESH, Uotila P, Jalonen JR, Nelenius H, Hartiala J, Yli-Mäyry S, Sheinin H. Relation of heart rate dynamics to the occurrence of myocardial ischemia after coronary artery bypass grafting. Am J Cardiol. 2002;89:1176–1191. doi: 10.1016/s0002-9149(02)02300-7. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Bigger JT, Jr, Goldsmith RL, Packer M. Effect of long-term digoxin therapy on autonomic function in patients with chronic heart failure. J Am Coll Cardiol. 1995;25:289–94. doi: 10.1016/0735-1097(94)00417-o. [DOI] [PubMed] [Google Scholar]


