Table 1.
Yields and conditions of Wittig reaction.
| Aldehyde | Phosphonium salt | Product (yield, %) |
|---|---|---|
| 2a |
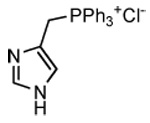 (3a) (3a) |
HYG (5)[a] |
| 2a | 4-(TBSO)C6H4CH2PPh3Br (3b) | YYG (23)[a] |
| 2a | BnPPh3Br (3c) | FYG (53)[b] |
| 2a | H2NCOCH2PPh3Br (3d) | NYG (49)[b] |
| 2a |
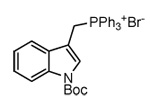 (3e) (3e) |
WYG (18)[b,c] |
| 2b | 4-(TBSO)C6H4CH2PPh3Br (3b) | YWG (42)[b] |
| 2b | BnPPh3Br (3c) | FWG (44)[b] |
2M aqueous NaOH / CH2Cl2.
t-BuOK / t-BuOH.
After Boc cleavage (see Experimental Section).
