Figure 3.
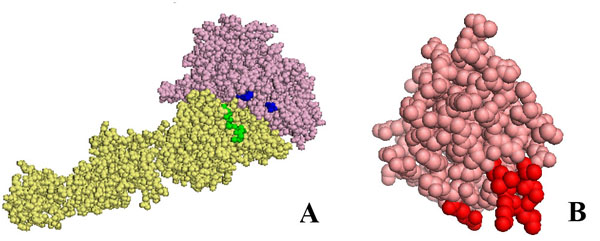
Residues of αIIbβ3 in the open conformation and of urokinase kringle domain which are differentially exposed upon binding. In panel A the α and β chains of αIIbβ3 are shown as pale pink and yellow spheres, respectively. Residues whose accessibility to the solvent changes by more than 10% upon kringle binding to αIIβ3 in the open form are highlighted in blue or green in the α chain or β chain respectively.
In panel B the kringle domain of urokinase is shown as salmon spheres. Residues whose accessibility to the solvent changes by more than 10% upon binding onto αIIbβ3 in open form are highlighted in red. In any case, only the residues differently exposed in all poses ranking second, third, fourth and fifth are highlighted.
