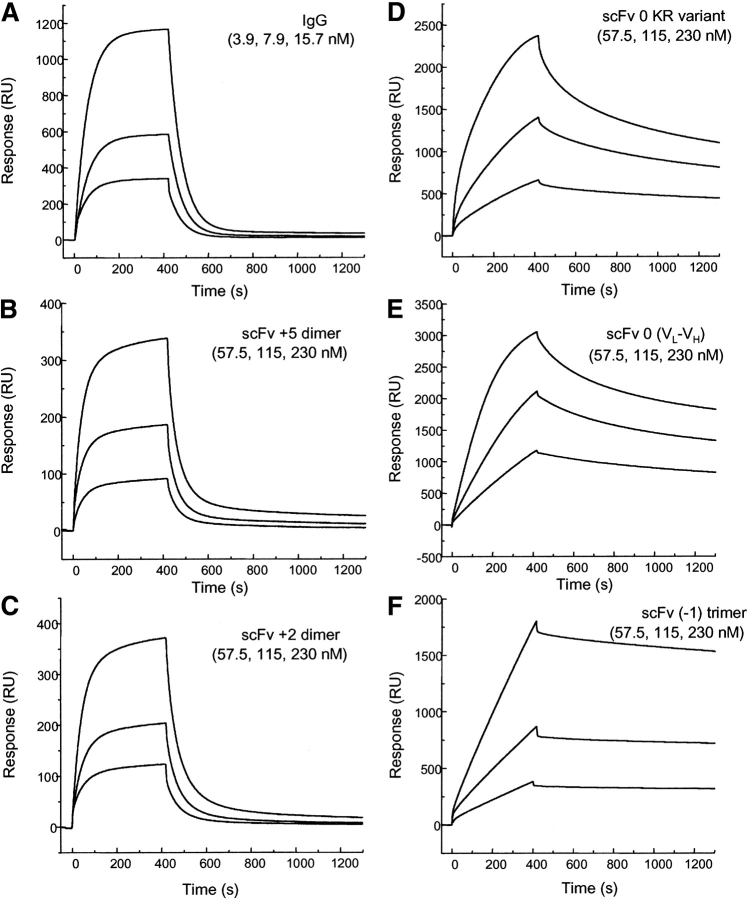
Figure 8.
Sensorgrams illustrating the binding hu3S193 scFv multimers to immobilized synthetic Ley tetrasaccharide-BSA complex (2100 RU) at a constant flow rate of 5 μL/min with an injection volume of 35 μL. The surface was regenerated with 10 μL of 100 mM HCl after each cycle. (A) hu3S193 IgG (3.9, 7.9, 15.7nM), (B) hu3S193 +5 dimer (57.5, 115, 230nM), (C) hu3S193 +2 dimer (57.5, 115, 230nM), (D) VH-0-VL KR variant trimer (57.5, 115, 230nM), (E) reverse VL-0-VH trimer/tetramer (57.5, 115, 230nM), (F) hu3S193 (−1) trimer (57.5, 115, 230 nM). Monomeric forms (hu3S193 +15 and Fab) not shown, and dimeric forms (IgG and hu3S193 +5 and hu3S193 +2) exhibit monovalent binding with the surface whereas hu3S193 trimer and tetramer show multivalent binding as indicated by the dissociation rates.