Figure 7.
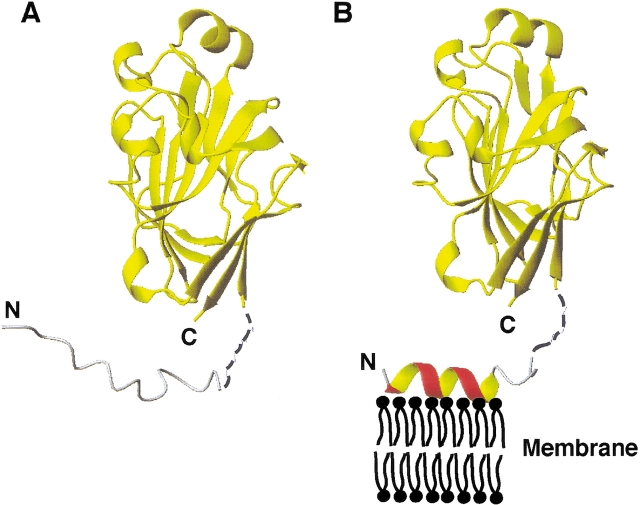
The two states of enzyme IIAGlc involved in the phosphoryl transfer cascade for the phosphorylation of glucose transported into E. coli. In the first state (A), the N-terminal domain of IIAGlc is disordered in solution ( Pelton et al. 1991) (gray) and not required for interaction with HPr (Wang et al. 2000a). In the second state (B), residues Leu 2–Val 10 of the N-terminal domain change conformation to an amphipathic helix (red), which functions as a membrane anchor to enhance the stability of the total IICBGlc-IIAGlc complex required for efficient phosphoryl transfer between them ( Wang et al. 2000b). Both the hydrophobic and cationic side chains (Lys 5 and Lys 7) in the amphipathic helix are required for the binding of the membrane anchor (red) to the E. coli membrane (black). Shown here is one of the possible orientations of the anchor on the membrane surface generated from the calculations. The coordinates for residues Asp16Thr17Gly18 are represented by broken lines (black) to reflect the fact that they were not observed in the crystal (Feese et al. 1997), nor determined in this study. In both states, the C-terminal domain, represented by a yellow ribbon, remains folded. The N- and C-termini of the protein are labeled. Figures 5 ▶ and 7 ▶ were made using the program MOLMOL (Koradi et al. 1996).