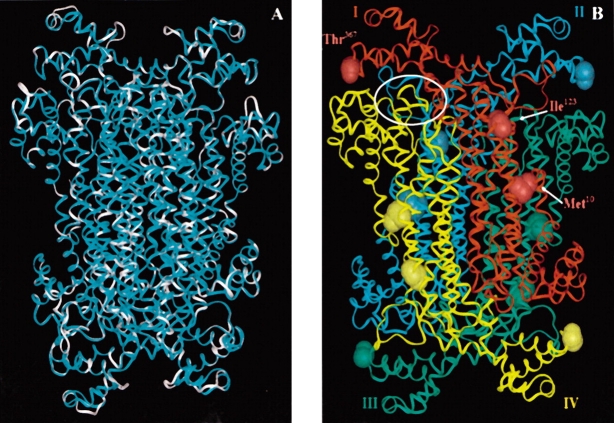
Figure 1.
(A) An overlay of the B. subtilis adenylosuccinate lyase (cyan) and the human enzyme (white) models. Calculating from the α carbons, the two structures have an RMS value of 0.3 Å. (B) Homology model of B. subtilis adenylosuccinate lyase based upon the Thermotoga maritima crystal structure, PDB 1c3c (Toth and Yeates 2000; Brosius and Colman 2002). On each of the four subunits (labeled I–IV and colored red, cyan, green, and yellow, respectively), the B. subtilis residues Met10, Ile123, and Thr367, corresponding to human enzyme mutations, have been displayed. One of the four active site regions has been encircled in white. There is an active site at each intersection of three subunits.