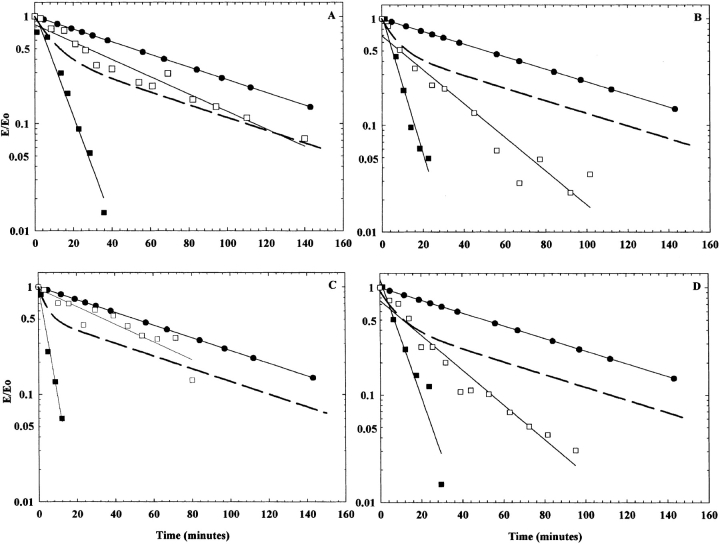
Figure 6.
Thermostability complementation studies at 42.5°C. In all panels, the wild type is represented by filled circles, and the theoretical line representing no complementation is dashed. (A) Wild type, M10L (filled squares), and M10L and WT (open squares) hybrid enzyme. (B) Wild type, I123R (filled squares), and I123R and WT (open squares) hybrid enzyme. (C) Wild type, I123W (filled squares), and I123W and WT (open squares) hybrid enzyme. (D) Wild type, T367R (filled squares) and T367R and WT (open squares) hybrid enzyme. The T367S enzyme is not shown, as it is as stable as the wild-type enzyme. The enzyme activities are expressed as E/E0 (observed activity/initial activity).