Figure 8.
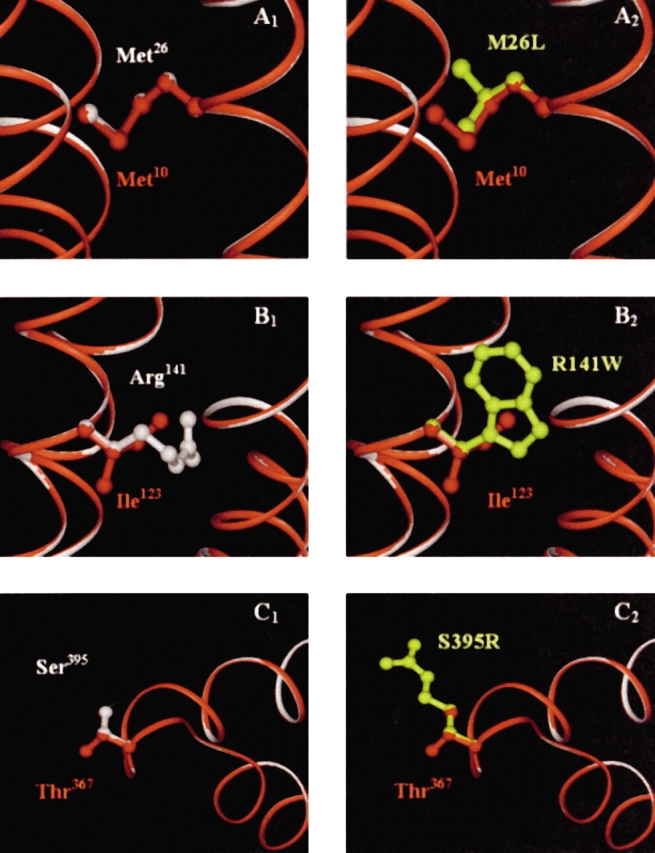
(A–C) An overlay of the human and B. subtilis enzyme homology models. The B. subtilis polypeptide backbone is shown in red, while the human backbone is in white. In (A1), Met10 of B. subtilis (red) and the corresponding wild-type residue in human, Met26 (white) are shown to completely superimpose. (A2) Shows the amino acid substitution to leucine (yellow) in the defective human enzyme. (B1) Shows Ile123 (B. subtilis, red) and Arg141 (wild-type human, white), which overlap through the β-carbons of both arginine and isoleucine. Replacement by a tryptophan (yellow) in the defective human enzyme is shown in (B2). Thr367 (B. subtilis, red) and Ser395 (wild-type human, white) are shown in (C1). These residues completely overlay, except for the additional methyl group of threonine. (C2) Shows the substitution to an arginine (yellow) in the defective human enzyme. The backbones of the two enzymes are completely superimposable in these regions.
