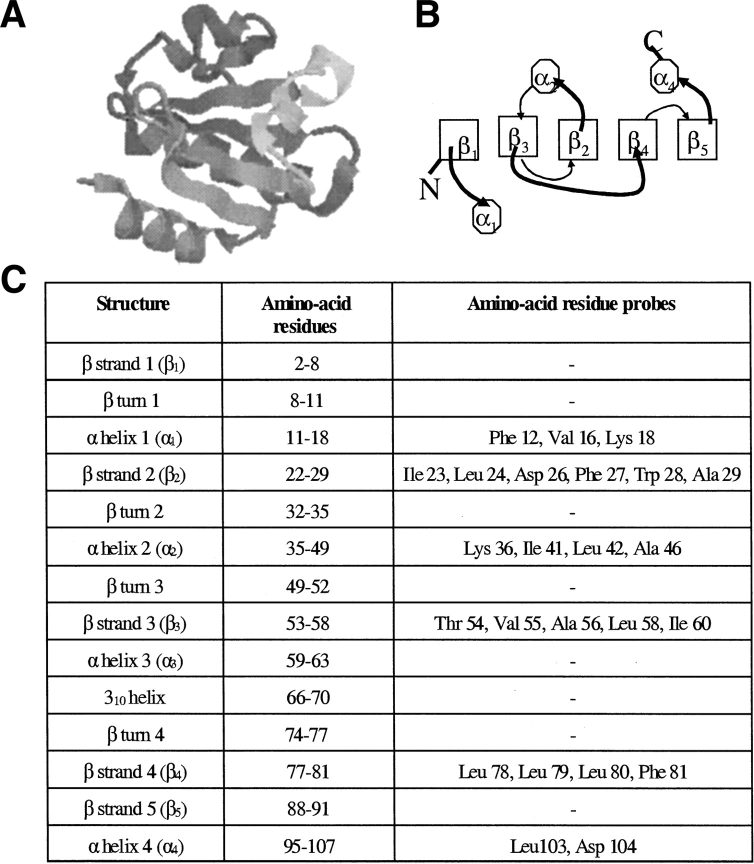
Figure 1.
Structure of Escherichia coli thioredoxin. (A) The crystal structure of thioredoxin (Katti et al. 1990) at 1.68 Å reveals two distinct domains: a large βαβαβ domain and a small ββα domain, joined by a segment consisting of a single turn α helix and a 310 helix. The figure has been generated using RASMOL (Sayle and Milner-White 1995) from the protein crystal structure 2TRX in the PDB, deposited by Katti et al. (B) Schematic representation of the topology of Trx, viewed down the axis of the β-sheet (Dyson et al. 1989). Boxes represent β strands, octagons represent α-helices, curved thick lines represent loops above, and curved thin lines represent loops below the β-sheet, respectively. The two subdomains are packed together via the β2 and β4 strands. (C) Location of the β strands, α-helices, and β-turns (Dyson et al. 1989) in Trx. The table also shows the amino acid residues whose backbone amide protons could be followed in this study.