Figure 1.
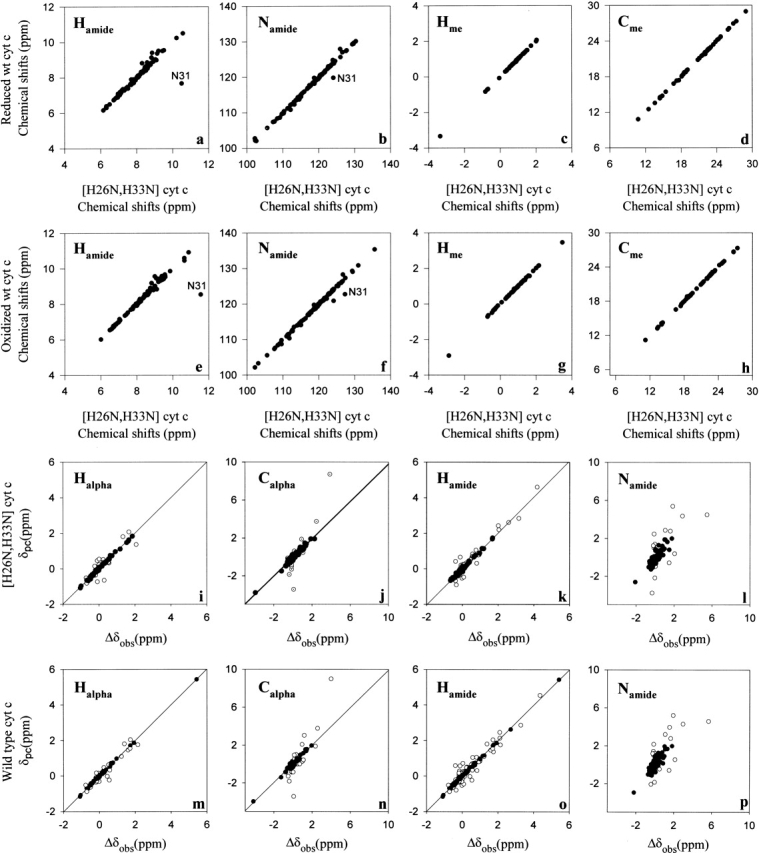
Comparison of the solution structures of natural wild-type horse cytochrome c and recombinant [H26N, H33N] horse cytochrome c. Correlation of the chemical shifts of reduced natural wild-type horse cytochrome c with those of reduced recombinant [H26N, H33N] horse cytochrome c (panel a, amide hydrogens; b, amide nitrogens; c, methyl protons; d, methyl carbons). Correlation of the chemical shifts of oxidized natural wild-type horse cytochrome c with those of oxidized recombinant [H26N, H33N] horse cytochrome c (panel e, amide hydrogens; f, amide nitrogens; g, methyl protons; h, methyl carbons). Refinement of the electronic g-tensor parameters for recombinant [H26N, H33N] horse cytochrome c (panels i–l) and natural wild-type horse cytochrome c (panels m–p). Solid circles correspond to those sites used to determine the parameters of the g-tensor by minimizing the difference between the observed and calculated pseudocontact shifts essentially as described by Feng et al. (1990). A similar plot is shown for recombinant [H26N, H33N] horse cytochrome c in panels m–p.
