Abstract
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms found in the gastrointestinal tract. The purpose of this study was to evaluate whether morphometric measurements could complement tumor size and mitotic activity in risk evaluation. Nuclear roundness and ellipse axis ratio were found to correlate with tumor size, mitotic activity, nuclear atypia, and hemorrhage. Morphometric variables in 422 GISTs were significant for overall survival in univariate analyses but did not retain independent significance in multivariate analyses incorporating mitotic count and tumor size. Traditional variables, together with sex, location of primary tumor, and nuclear atypia, seem to be the best parameters for prognostic evaluation. (J Histochem Cytochem 56:139–145, 2008)
Keywords: gastrointestinal stromal tumor, morphometry, roundness
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms arising in the digestive tract (Miettinen et al. 1998; Steigen and Eide 2006). Such tumors arise predominantly in the stomach (50%) and small intestine (25%) but can be found throughout the gastrointestinal tract. The age of patients with GISTs range from young adults to older than 90 years of age, but peak incidence is at ∼60 years of age. Most GISTs express the protein kinase KIT, and its immunohistochemical marker CD117 is important for making the diagnosis (Miettinen and Lasota 2001; Fletcher et al. 2002) by excluding other soft tissue tumors such as leiomyomas, leiomyosarcomas, schwannomas, and inflammatory polyps.
Accurate diagnosis and evaluation of malignant risk is very important for the treatment of patients with GISTs. Standard treatment for localized GISTs is complete surgical resection. In more advanced disease, the tumor might not be resectable, may be only partly resectable, or be resectable only at the cost of significant morbidity. In patients with tumors regarded as highly malignant or recurrent, metastatic, and/or unresectable, the kinase inhibitor imatinib mesylate (Glivec) is given, and this treatment has improved the outcome for these patients (van Oosterom et al. 2001; Demetri et al. 2002). The greater problem with GISTs is determining risk of malignancy and, by extension, the need for aggressive surgical and potentially expensive medical intervention. GISTs with low malignant potential, in contrast, can be marginally excised or simply monitored for growth. Factors for calculation of malignant risk in GISTs are controversial, but the most accepted pathologic features are mitotic rate and tumor size (Fletcher et al. 2002) Other factors such as anatomic location, cellular atypia, and necrosis have been shown to be independent prognostic factors (Miettinen et al. 2005).
One way to overcome the potential problem associated with subjective histological assessments of mitotic activity and nuclear atypia is to develop more objective and reproducible analytical techniques to quantify key histopathologic features. Digital image analysis can provide an objective assessment of nuclear morphology to complement conventional histopathology. Assessment of nuclear morphometry has been used in several studies in breast cancer for prediction of recurrence (Hoque et al. 2001; Tan et al. 2001). Recent studies have shown that there is a relationship between morphometric measurements expressed as the D-score (discriminate score) and the prognosis of endometrial hyperplasia, (Ausems et al.1985; Baak et al. 2001). Partin et al. (1989) evaluated usefulness of nuclear morphology for prognostication in stage A2 prostate cancer in 255 patients and found that an average nuclear roundness factor provided more significant separation of patient outcome than the Gleason score did.
Cunningham et al. (1993) predicted prognosis of 122 gastrointestinal smooth muscle tumors (SMTs) based on clinical and histological evaluation, flow cytometry, morphometry, and image cytometry. Immunohistochemical assays were used to ascertain that the tumors were not from neurogenic derivation. From our knowledge today, many of these tumors are likely to have been GISTs (Steigen and Eide 2006). Using morphometric techniques, they assessed 100 tumor cell nuclei in each case. None of the morphometric measurements (nuclear perimeter, nuclear area, nuclear circularity, longest nuclear diameter, nuclear average Feret diameter, and nuclear equivalent diameter) were significant when analyzed using a univariate Cox proportional hazard model.
The purpose of this study was to determine if morphometric measurements could complement clinical, macroscopic, and microscopic findings to predict the biological behavior of GISTs. Morphometric calculations have been incorporated in evaluation of prognosis in other neoplasms and also on what was previously known as gastrointestinal SMTs. Our diagnoses are more precise today with the use of new antibodies (particularly CD117). Image analysis technology has also significantly improved. In this study, we use digital image morphometry to analyze a large series of well-characterized GISTs with accessible clinical and histologic information. We explore the correlation of morphometric factors with clinical and morphologic characteristics of the patients, and the potential prognostic value of these morphometric factors in univariate and in multivariate analyses including established prognostic factors in GISTs.
Materials and Methods
Patients and Samples
The study material consisted of selected tissue blocks from the archives of pathology departments throughout Norway. Cases were selected by evaluating the records of the Cancer Registry of Norway for mesenchymal tumors and poorly differentiated carcinomas in the gastrointestinal tract over a period of 30 years (1973–2002). A total of 3672 reports were evaluated, and all reports with clear evidence of origin of tumor not being mesenchymal were discarded (based on the results of immunohistochemical staining and other methods reported at the time of primary diagnosis). A total of 1192 cases of candidate mesenchymal tumors were identified, and slides and blocks from all these cases were requested. The material was located in all of the 20 pathology departments in Norway. From two hospitals, we received no material, which constituted 92 cases. In 231 cases, no blocks were found or the blocks did not contain enough material for further study. From the archives of the Department of Pathology at the University Hospital of Northern Norway, an additional 64 cases of possible mesenchymal tumors were retrieved. New slides of the remaining 933 cases were made and stained with hematoxylin and eosin (H&E) and histologically reexamined by gastrointestinal (SES) and mesenchymal tumor (TON) subspecialty pathologists. This excluded an additional 159 cases from the study because they were almost certainly not mesenchymal tumors (based on the H&E stain) but rather carcinomas or lymphomas. Seven hundred seventy-four cases were evaluated as representing true mesenchymal tumors of the gastrointestinal tract, but for making tissue microarrays (TMAs), another 68 cases lacked sufficient material for the required duplicate core extractions (blocks containing only small bite or core biopsies). The 706 remaining cases were assembled into five TMAs. Only eight patients were treated with STI-571 (Glivec; Novartis, Basel, Switzerland), because this cohort largely predates the use of this drug. The Regional Committee for Medical Research Ethics, Northern Norway, approved the study.
Construction of Tissue Microarrays
The slide with representative, viable tumor was selected from each case and coupled to the corresponding formalin-fixed paraffin-embedded block. Duplicate 0.6-mm cores were taken from representative tumor tissue and inserted into a recipient paraffin block to create a tissue microarray, using a Beecher Instruments (Silver Spring, MD) MicroTissue Arrayer (Parker et al. 2002). The completed recipient blocks were sectioned at 4 μm and transferred to silanized glass slides. A total of five recipient blocks were made.
Histological Evaluation
The whole section slides that were used for selecting representative TMA cores were used for histological evaluation of morphologic characteristics. Mitotic figures were counted in 50 consecutive high-power fields (HPFs). Additional variables recorded included the presence of spindled and/or epithelioid cell morphology. Focal or diffuse atypia was defined according to Miettinen et al. (2006). Other variables such as necrosis, ulceration, and hemorrhage were also assessed.
Immunohistochemical Staining and Scoring
Sections from the arrays were stained with H&E to confirm the presence of representative tumor in each core. Further sections were stained using a Ventana (Tucson, AZ) automated immunohistochemical stainer according to manufacturer's guidelines. The antibody used for detecting KIT-positive cases was c-KIT (polyclonal, dilution 1:200; Dako, Carpinteria, CA). The immunostaining was performed with an avidin-biotin detection system. Diaminobenzidine hydrochloride solution with hydrogen peroxide (Ventana Gen II, Dab basic) was the chromogen.
Morphometric Scoring
Digital images of sections from the TMA stained with H&E were used for morphometric analyses, captured using a BLISS scanner (Bacus Laboratories; Lombard, IL). The slides were scanned at ×40 objective magnifications. A plug in for the open source program ImageJ (http://rsb.info.nih.gov/ij/) was created to combine the separate images stored by BLISS scanner and form the individual images of the tissue cores (http://rsb.info.nih.gov/ij/plugins/stitch-bliss.html). The resulting pixel dimensions of the individual core images were 3760 × 3360. A separate Nuclear Stain Analyzer plug in was created for assessment of morphometric parameters of the tumor nuclei (Figure 1). The following characteristics/dimensions of the nuclei were thereby quantified: area, intensity, optical density, perimeter, width and height of bounding rectangle, ellipse major and minor, ellipse ratio, ellipse angle, circularity, Feret diameter, and roundness. In addition, number of nuclei in each core was registered. The formula used for roundness was 4 × area/π × square(major axis). Ellipse major and minor were the primary and secondary axes of best-fitting ellipse, and the ratio between shortest and longest axis was used to evaluate the ratio between the two (SL ratio).
Figure 1.
Core with outlined nuclei.
All the images are publicly available at the companion site: www.gpecimage.ubc.ca/tma/web/viewer.php. The site was constructed at Genetic Pathology Evaluation Centre (GPEC) (Vancouver, Canada) using a GPEC database and a Java applet provided by Bacus Laboratories.
Clinical and Morphologic Factors
Clinical factors included sex, age at diagnosis, month and year of diagnosis, and location of primary tumor. All these variables were evaluated as prognostic factors in univariate and multivariate analyses. There was no record of cause of death.
Morphologic factors including size of tumor, number of mitosis, spindle or epiteloid appearance of tumor (or both), nuclear atypia (focal, diffuse, or none), ulceration, hemorrhage, coagulative or liquefying necrosis, and infiltration into mucosa or muscle were all correlated with the morphometric parameters listed above. They were all evaluated as prognostic factors in univariate and multivariate analyses.
Statistics
Data were analyzed using SPSS (14.0; SPSS, Chicago, IL). Variance components estimation was used to evaluate difference in number of nuclei between duplicate cores from each case.
We first studied the associations between traditional morphologic variables and morphometric measures and used independent t-test or one-way ANOVA for comparison of means and correlation coefficient to indicate the relation among continuous variables. Finally, a multiple linear regression analysis was performed to single out independent associations. The further statistical analysis was built on the following reasoning. Morphometric measurements are putative predictors of grade of malignancy, and thereby of survival, and might add to the predictive value of traditional morphologic characteristics. We first established which morphologic and morphometric variables were significantly associated with survival by univariate Cox regression. From a multivariate Cox regression model with the morphologic variables including all univariately significant variables, we added the morphometric variables one by one to evaluate their possible predictive ability. In the multivariate analysis, categorical entities are represented as either dichotomous or dummy variables. We did not apply any stepwise selections in our analysis. Kaplan-Meier curves are used for illustrative purpose.
Results
Four hundred forty-two cases from the TMAs stained positive for CD117 and were regarded as definite GISTs.
Clinical and Morphologic Characteristics
There were 229 men and 213 women, with a mean age of 64.5 and 65.5 years, respectively, at the time of diagnosis. Four hundred twenty-two cases were within the 30-year period of 1973–2002. Stratified into periods of 10 years (Table 1), there was a steady increase in the number of cases with a significant increase in the number of women (p=0.032).
Table 1.
Occurrence of gastrointestinal stromal tumor cases by calendar period and sex
| Sex
|
||||
|---|---|---|---|---|
| Female | Male | Total | ||
| Diagnosis per 10 years | 1973–1982 | 41 | 54 | 95 |
| 1983–1992 | 53 | 75 | 128 | |
| 1993–2002 | 105 | 94 | 199 | |
| Total | 199 | 223 | 422 | |
Location of the primary tumor was the stomach in 228 cases, small bowel in 152 cases, and other locations in 62 cases. One hundred thirty-six tumors were <5 cm and 193 were >5 cm. In 113 cases, there was no size reported. In 298 cases, the tumors had five mitoses or less per 50 HPFs and 144 had more than five mitoses. In 184 cases, hemorrhage was found, in 42 cases, ulceration was found, and in 41 cases, coagulative necrosis was found. Two hundred seventy-two tumors lacked significant nuclear atypia, 117 were regarded as having focal nuclear atypia, and 53 had diffuse nuclear atypia. A total of 326 tumors were classified as predominantly spindle cell morphology, 41 as predominantly epithelioid cells, and 75 as a combination of both.
Morphometric Characteristics
Four hundred twenty-two of the 442 KIT-positive GISTs had two cores available for morphometric studies. A total of 1,542,184 nuclei were measured, with 770,359 measured nuclei in core 1 and 771,825 measured in core 2 images, and a range between 529 and 11,207 nuclei were counted in each core. In a variance component analysis, the variation caused by the two cores was negligible.
The range of nuclear roundness was 0.2655–0.5113 (mean, 0.4061; SEM, 0.0020), the range of nuclear SL ratio (shortest axis divided on longest axis) was 0.3028–0.6824 (mean, 0.5694; SEM, 0.0027), and the range of the Feret diameter was 8.2421–25.0574 (mean, 10.1023; SEM, 0.0915). Table 2 shows the mean nuclear roundness, mean nuclear SL ratio, and mean number of nuclei by sex and in subgroups of the morphologic variables. Age did not correlate with the morphometric variables (data not shown).
Table 2.
Mean nuclear roundness and nuclear ratio in 422 patients with gastrointestinal stromal tumors, stratified by pathological variables
| N | Roundness, mean | Significance | Nuclear SL ratio, mean | Significance | Number of nuclei | Significance | |
|---|---|---|---|---|---|---|---|
| Size | 129 with 1–50 mm | 0.391 | <0.001 | 0.551 | <0.001 | 3200 | 0.001 |
| 184 with >50 mm | 0.413 | 0.579 | 3754 | ||||
| Mitoses | 279 with 0–5 mitoses | 0.396 | <0.001 | 0.558 | <0.001 | 3370 | <0.001 |
| 142 with >5 mitoses | 0.427 | 0.592 | 4059 | ||||
| Focal nuclear atypia | 261 with no atypia | 0.398 | 0.001 | 0.562 | 0.017 | 3590 | 0.815 |
| 112 with focal atypia | 0.415 | 0.577 | 3550 | ||||
| Diffuse nuclear atypia | 261 with no atypia | 0.398 | <0.001 | 0.562 | <0.001 | 3590 | 0.425 |
| 49 with diffuse atypia | 0.427 | 0.593 | 3784 | ||||
| Hemorrhage | 247 with no hemorrhage | 0.401 | 0.003 | 0.562 | 0.001 | 3559 | 0.494 |
| 175 with hemorrhage | 0.413 | 0.580 | 3662 | ||||
| Cell type | 318 with spindle cells | 0.403 | 0.001 | 0.564 | <0.001 | 3571 | 0.329 |
| 35 with epiteloid cells | 0.430 | 0.605 | 3423 | ||||
| 69 with spindle and epiteloid | 0.408 | 0.577 | 3834 | ||||
| Sex | 221 men | 0.407 | 0.68 | 0.570 | 0.973 | 3718 | 0.100 |
| 201 women | 0.405 | 0.569 | 3474 |
SL, shortest and longest axis.
Tumors with more than five mitoses per 50 HPFs had significantly rounder nuclei and increased mean nuclear SL ratio compared with tumors with fewer mitoses (p<0.001 for both parameters). Nuclear roundness and nuclear SL ratio were also statistically significant when comparing tumor size (>5 cm), focal nuclear atypia, diffuse atypia, and hemorrhage. No association was found in roundness or nuclear ratio with location (gastric or small bowel tumors), coagulative necrosis, or ulceration. There were no significant differences found for number of nuclei or mean Feret diameter for any of these variables. Variables such as area, intensity, optical density, perimeter, width and height of bounding rectangle, ellipse angle, and circularity were not found to be significant, and no further calculations with these were made.
In multiple linear regression models with mean roundness and mean SL ratio as dependent variables, tumor size (p=0.002 and 0.006), mitoses (p<0.001 in both), and hemorrhage (p=0.003 and 0.001) were significant. The model with nuclear roundness as the dependent variable was the most significant.
Survival Analyses
Younger patients (<50 years of age) had the longest median overall survival time (12 years), with a steady decrease to 7.3 years for patients 51–60 years of age, 3.8 for patients 61–70 years of age, and 3.1 for patients 71–80 years of age (p<0.001). Median survival time for men was 3.6 years and for women was 5.5 years, which is statistically significant (p=0.027). Patients with gastric tumors had an overall better survival than patients with tumors in the small bowel or any other location (p<0.001).
Patients with tumors with less than five mitoses per 50 HPFs had a median survival time of 6.5 years, whereas those with more than five mitoses have a median survival of 2.9 years (p<0.001). For size <5 and >5 cm, the corresponding data were 7.4 and 3.4 years (p<0.001). Median survival time for tumor without atypia was 5.4 years, focal nuclear atypia, 2.5 years, and diffuse nuclear atypia, 2.2 years (p<0.001). For patients with no identified coagulative necrosis, the median survival time was 4.4 years compared with 3.8 years for those with coagulative necrosis (p=0.006). In patients with ulceration, hemorrhage, and spindle or epithelioid cell types, there were no significant differences in overall survival.
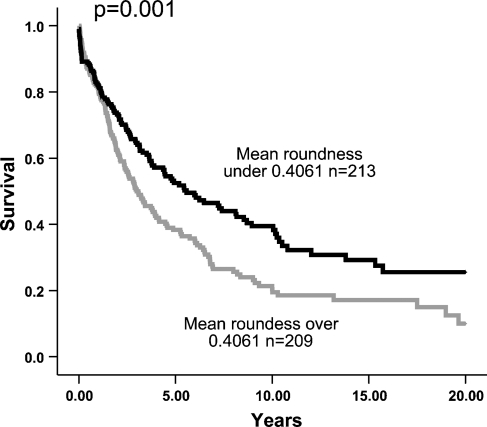
In a Cox univariate model, mean roundness (p<0.001), mean SL ratio (p=0.017), and number of nuclei (p=0.043) were significant predictors of overall survival, whereas Feret diameter was not (p=0.94) (Figure 2). When tumors were stratified according to site, mean roundness was a significant predictor of overall survival both in the gastric (p=0.032) and small intestinal GISTs (p=0.047).
Figure 2.
Survival of patients with median roundness less than and more than 0.4061 (mean value).
Mean roundness and nuclear SL ratio showed a strong correlation (0.919), and therefore, only one of these was used in multivariate Cox model calculations. Mean roundness was arbitrarily chosen. The result of the Cox proportional hazard analysis is presented in Table 3. The age at diagnosis was divided into decades, and the new values were used as continuous variables. Location of primary tumor was divided into three categories: site other than stomach or small bowl, gastric location, or location in the small bowel, with values of 0, 1, or 2, respectively. These were used as categorical values. Size of tumor was likewise divided into three categories with unknown size, size <5 cm, and size >5 cm and used as categorical values. Mean roundness and number of nuclei were not independently significant in a model including sex, age at diagnosis, location of primary tumor, size, mitoses, nuclear atypia, coagulative necrosis, and hemorrhage.
Table 3.
Cox proportional hazard regression analysis with demographic, morphologic, and morphometric variables
| Demographic
|
Morphologic
|
Morphometric
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | p | Hazard ratio | p | Hazard ratio | p | |
| Male sex | 1.292 | 0.039 | 1.307 | 0.32 | 1.299 | 0.37 |
| Age at diagnosis | 1.404 | <0.001 | 1.410 | <0.001 | 1.412 | <0.001 |
| Location of primary tumor (gastric or nongastric) | 1.549 | 0.002 | 1.542 | 0.002 | 1.565 | 0.002 |
| Size | 1.424 | 0.030 | 1.385 | 0.046 | 1.374 | 0.53 |
| Mitoses | 1.995 | <0.001 | 1.870 | <0.001 | 1.846 | <0.001 |
| Nuclear atypia | 1.231 | 0.022 | 1.228 | 0.024 | 1.240 | 0.20 |
| Coagulative necrosis | 1.412 | 0.098 | 1.458 | 0.072 | 1.452 | 0.076 |
| Hemorrhage | 0.873 | 0.285 | 0.839 | 0.177 | 0.844 | 0.191 |
| Mean roundness | 1.279 | 0.139 | 1.249 | 0.202 | ||
| Number of nuclei | 1.000 | 0.630 | ||||
Discussion
Number of mitoses and tumor size are currently regarded as the most helpful variables for evaluation of malignancy of GISTs, and the results of our study confirm their influence on overall survival in our series of 442 cases from Norway. This supports the usefulness of the classification according to a consensus risk group stratification system based on maximum tumor size and mitotic count (Fletcher et al. 2002).
Many techniques have been proposed for accurately predicting prognosis for GISTs. Chromosomal aberrations with loss of chromosomes 9 and 1 have been found to be quite specific for malignant GISTs (Debiec-Rychter et al. 2001). Gain of chromosome 8 and loss of chromosomes 7 and 15 also indicated malignant GISTs. DNA ploidy has also been used to objectively determine biological behavior and prognosis of solid neoplasias. In a study by Carrillo et al. (1997), DNA aneuploidy and high proliferation index (measured by MIB-I) correlated significantly with patient outcome. Also, Cunningham et al. (1993) found aneuploidy to be a significant predictor of mortality in an univariate model. These techniques are found to be both labor intensive and expensive, and none were proven to hold up in multivariate analyses, including mitotic count.
Many pathologists now have their microscopes coupled to high-technology digital cameras and computers. This allows the investigator to capture digital images that can aid in the diagnosis of the individual case. Morphometric study has been regarded to be a time-consuming and expensive method, but this is not necessarily true. Measurement of different variables using digital analyses could be a complimentary method in the evaluation of tumor material without being biased by the potentially subjective interpretation of the individual investigator. The malignancy risk of GISTs could be calculated by the pathologist based on a summary of both traditional criteria and morphometry. This in turn could help clinicians ensure that patients receive the most appropriate treatment. Bearing this in mind, such new techniques are important to validate, preferably on large tumor series. A paper on nuclear morphometry on GISTs has been done on a small series of samples (22 cases), and the data from this preliminary study suggest value in computer-assisted image analysis (Ozdamar et al. 2007).
When exploring the correlation of morphologic characteristics to morphometric factors, tumor size, number of mitoses, nuclear atypia, and hemorrhage were all significant. In the study by Ozdamar et al. (2007), necrosis and mitotic index were found to correlate and not tumor size. The reason for the somewhat concurrent results might be the small sample size in the latter study. This does, however, show the interest and importance of such analysis in GISTs.
Our results on prognostic factors validate some previously reported data, but new variables should also be considered. Size of tumor and number of mitoses are regarded to be among the most reliable variables for predicting prognosis (Fletcher et al. 2002), and this was confirmed in our study. Nuclear roundness and SL ratio were found to correlate very well with both mitoses and tumors size. The more elongated nuclei were found in the tumors with fewer mitoses and smaller size. In this setting, it was interesting to test if the morphometric characteristics would remain significant for overall survival in a multivariate model, exceeding and/or displacing mitotic count and size. They failed, however, to do so. Indeed, mitotic count, size, sex, patient age, anatomical location, and nuclear atypia were the most powerful predictors of overall survival in multivariate models, rendering morphometric parameters insignificant.
Women proved to have a more favorable overall outcome than men in the series. Sex has not been found to be of significance in population-based studies from Sweden and Iceland (Nilsson et al. 2005; Tryggvason et al. 2005), but in a study by DeMatteo et al. (2000), male sex was a poor prognostic sign. The mean age at the time of diagnosis was almost equal between the sexes, and reasons other than age for longer survival among the women in our study must be considered. The location of the primary tumor has been found to be of importance by others (Emory et al. 1999; Nilsson et al. 2005) and is again confirmed in this study. Patients with gastric GISTs lived longer than patients with small bowel tumors. Nuclear atypia, either focal or diffuse, was found in this series to be an unfavorable morphologic variable, which has previously been reported by some (Miettinen et al. 2005) but not by others (Iesalnieks et al. 2005).
In this study, we tested nuclear morphometric characteristics as potential independent variables for evaluating risk of malignancy in a large series of GISTs. In univariate models, some of these factors show a prognostic value for survival, but the significance is lost in multivariate models because of the strong correlation with mitoses and tumor size. Nuclei with less round features and with a lower SL ratio correspond to spindle cells, and those with rounder nuclei and higher SL ratios correspond to epithelioid cells. Investigation of predominant cell type, spindled or epithelioid, in GISTs has been evaluated in our study and also by others, without showing significance for overall survival (Carrillo et al. 1997; Iesalnieks et al. 2005; Miettinen et al. 2005).
Nuclear roundness and nuclear ratio are interesting independent variables but should probably not be considered important as prognostic variables at this stage. Anatomical site of the primary tumor is now being accepted as a variable to be considered in risk stratification of primary GISTs (Hornick and Fletcher 2007). We suggest, however, that sex and nuclear atypia should also be considered when risk of malignancy in GISTs is evaluated. Further study of variables, which may provide information beyond that, gleaned from number of mitoses, tumor size, and site, is required in the prognostication of GISTs.
Acknowledgments
T.O.N. is a Scholar of the Michael Smith Foundation for Health Research. The tissue microarray facility at the Genetic Pathology Evaluation Centre is supported in part by an unrestricted educational grant from sanofi-aventis.
References
- Ausems EW, van der Kamp JK, Baak JP (1985) Nuclear morphometry in the determination of the prognosis of marked atypical endometrial hyperplasia. Int J Gynecol Pathol 4:180–185 [DOI] [PubMed] [Google Scholar]
- Baak JP, Ørbo A, van Diest PJ, Jiwa M, de Bruin P, Broeckaert M, Snijders W, et al. (2001) Prospective multicenter evaluation of the morphometric D-score for prediction of the outcome of endometrial hyperplasias. Am J Surg Pathol 25:930–935 [DOI] [PubMed] [Google Scholar]
- Carrillo R, Candia A, Rodriguez-Peralto JL, Caz V (1997) Prognostic significance of DNA ploidy and proliferative index (MIB-1 index) in gastrointestinal stromal tumors. Hum Pathol 28:160–165 [DOI] [PubMed] [Google Scholar]
- Cunningham RE, Federspiel BH, McCarthy WF, Sobin LH, O'Leary TJ (1993) Predicting prognosis of gastrointestinal smooth muscle tumors. Role of clinical and histologic evaluation, flow cytometry, and image cytometry. Am J Surg Pathol 17:588–594 [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter M, Lasota J, Sarlomo-Rikala M, Kordek R, Miettinen M (2001) Chromosomal aberrations in malignant gastrointestinal stromal tumors: correlation with c-KIT gene mutation. Cancer Genet Cytogenet 128:24–30 [DOI] [PubMed] [Google Scholar]
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, et al. (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480 [DOI] [PubMed] [Google Scholar]
- Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ (1999) Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol 23:82–87 [DOI] [PubMed] [Google Scholar]
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, et al. (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33:459–465 [DOI] [PubMed] [Google Scholar]
- Hoque A, Lippman SM, Boiko IV, Atkinson EN, Sneige N, Sahin A, Weber DM, et al. (2001) Quantitative nuclear morphometry by image analysis for prediction of recurrence of ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev 10:249–259 [PubMed] [Google Scholar]
- Hornick JL, Fletcher CD (2007) The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol 38:679–687 [DOI] [PubMed] [Google Scholar]
- Iesalnieks I, Rummele P, Dietmaier W, Jantsch T, Zulke C, Schlitt HJ, Hofstadter F, et al. (2005) Factors associated with disease progression in patients with gastrointestinal stromal tumors in the pre-imatinib era. Am J Clin Pathol 124:740–748 [DOI] [PubMed] [Google Scholar]
- Miettinen M, Lasota J (2001) Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 438:1–12 [DOI] [PubMed] [Google Scholar]
- Miettinen M, Makhlouf H, Sobin LH, Lasota J (2006) Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 30:477–489 [DOI] [PubMed] [Google Scholar]
- Miettinen M, Sarlomo-Rikala M, Lasota J (1998) Gastrointestinal stromal tumours. Ann Chir Gynaecol 87:278–281 [PubMed] [Google Scholar]
- Miettinen M, Sobin LH, Lasota J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52–68 [DOI] [PubMed] [Google Scholar]
- Nilsson B, Bumming P, Meis-Kindblom JM, Oden A, Dortok A, Gustavsson B, Sablinska K, et al. (2005) Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era–a population-based study in western Sweden. Cancer 103:821–829 [DOI] [PubMed] [Google Scholar]
- Ozdamar SO, Bektas S, Ozdamar SE, Gedikoglu G, Gun BD, Bahadir B (2007) Nuclear morphometric analysis in gastrointestinal stromal tumors: a preliminary study. Turk J Gastroenterol 18:71–76 [PubMed] [Google Scholar]
- Parker RL, Huntsman DG, Lesack DW, Cupples JB, Grant DR, Akbari M, Gilks CB (2002) Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol 117:723–728 [DOI] [PubMed] [Google Scholar]
- Partin AW, Walsh AC, Pitcock RV, Mohler JL, Epstein JI, Coffey DS (1989) A comparison of nuclear morphometry and Gleason grade as a predictor of prognosis in stage A2 prostate cancer: a critical analysis. J Urol 142:1254–1258 [DOI] [PubMed] [Google Scholar]
- Steigen SE, Eide TJ (2006) Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS 114:192–200 [DOI] [PubMed] [Google Scholar]
- Tan PH, Goh BB, Chiang G, Bay BH (2001) Correlation of nuclear morphometry with pathologic parameters in ductal carcinoma in situ of the breast. Mod Pathol 14:937–941 [DOI] [PubMed] [Google Scholar]
- Tryggvason G, Gislason HG, Magnusson MK, Jonasson JG (2005) Gastrointestinal stromal tumors in Iceland, 1990–2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 117:289–293 [DOI] [PubMed] [Google Scholar]
- van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato DP, Dimitrijevic S, Martens M, et al. (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358:1421–1423 [DOI] [PubMed] [Google Scholar]