Abstract
The predictive and prognostic value of fluorodeoxyglucose (FDG)-positron emission tomography (PET) in non-small-cell lung carcinoma, colorectal carcinoma and lymphoma is discussed. The degree of FDG uptake is of prognostic value at initial presentation, after induction treatment prior to resection and in the case of relapse of non-small cell lung cancer (NSCLC). In locally advanced and advanced stages of NSCLC, FDG-PET has been shown to be predictive for clinical outcome at an early stage of treatment. In colorectal carcinoma, limited studies are available on the prognostic value of FDG-PET, however, the technique appears to have great potential in monitoring the success of local ablative therapies soon after intervention and in the prediction and evaluation of response to radiotherapy, systemic therapy, and combinations thereof. The prognostic value of end-of treatment FDG-PET for FDG-avid lymphomas has been established, and the next step is to define how to use this information to optimize patient outcome. In Hodgkin's lymphoma, FDG-PET has a high negative predictive value, however, histological confirmation of positive findings should be sought where possible. For non-Hodgkin's lymphoma, the opposite applies. The newly published standardized guidelines for interpretation formulates specific criteria for visual interpretation and for defining PET positivity in the liver, spleen, lung, bone marrow and small residual lesions. The introduction of these guidelines should reduce variability among studies. Interim PET offers a reliable method for early prediction of long-term remission, however it should only be performed in prospective randomized controlled trials. Many of the diagnostic and management questions considered in this review are relevant to other tumour types. Further research in this field is of great importance, since it may lead to a change in the therapeutic concept of cancer. The preliminary findings call for systematic inclusion of FDG-PET in therapeutic trials to adequately position FDG-PET in treatment time lines.
Keywords: FDG-PET, prediction, prognosis, response monitoring, therapy monitoring, SUV, non-small-cell lung cancer, colorectal cancer, lymphoma
Introduction
For several years, [18F]fluorodeoxyglucose (FDG)-positron emission tomography (PET) has become part of the standard of care in pre-surgical staging of a variety of malignant diseases, focusing on the detection of malignant lesions at early stages and early detection of recurrence and metastatic spread. FDG-PET has been shown to be successful in distinguishing fibrosis and scar from viable tumour in residual masses after therapy and in localization of recurrence in patients with an unexplained rise of serum tumour markers. FDG-PET has a positive impact on overall staging and patient management for a wide variety of malignancies. However, there is more beyond staging. The metabolic information provided by FDG-PET makes this technique a promising imaging biomarker. Biomarkers in medical practice have been defined as indicators of normal biological or pathologic processes, or indicators of response to treatment and they can serve as a surrogate clinical endpoint. Thus, by definition, many specimen features can be considered biomarkers. The strength of FDG-PET, however, is that it permits whole-body imaging in a non-invasive way. It is therefore not limited to characterizing one or a few target lesions, but can evaluate multiple tumour sites at the same time. Furthermore, serial scanning can be performed, which allows for measurement of functional changes over time during therapeutic interventions. The technique can visualize and quantify FDG uptake and is able to provide several highly reproducible quantitative parameters of tumour glucose metabolism. As individualized treatment strategies become more relevant and the choice of anti-tumour agents is expanding considerably, there is growing interest in the use of FDG-PET as a response-monitoring tool. Some new therapies may be cytostatic instead of cytoreductive, in which case successful treatment may not lead to a decrease in tumour size, which poses new demands on imaging modalities. The present review aims to discuss the predictive and prognostic value of FDG-PET. It addresses the role of FDG-PET in identifying tumour response to anti-cancer therapies and in identifying subsets of patients with poor outcome among patients with non-small-cell lung cancer (NSCLC), colorectal carcinoma and lymphoma.
Prognostic value of FDG-PET in NSCLC
The introduction of the combined use of FDG-PET and computed tomography (CT) has had a major impact on the diagnosis and staging of lung cancer. FDG-PET has been employed to evaluate unclassified pulmonary nodules for malignancy[1],[2]. Furthermore, it provides non-invasive mediastinal staging and reduces the number of futile thoracotomies and mediastinoscopies[3–5]. This imaging modality detects unsuspected extrathoracic metastases in 14–17% of patients otherwise considered potentially resectable[6].
Recently, FDG-PET has also demonstrated its value in radiation treatment planning and detection of recurrent disease[7–10]. Currently, there is an increasing interest in the role of FDG-PET beyond staging, such as the evaluation of biological characteristics of the tumour and prediction of prognosis in the context of treatment stratification and the early assessment of tumour response to therapy. To date, the tumour–node–metastasis (TNM) staging system is considered the most important tool to estimate prognosis and the most important guide in treatment decisions[11]. However, the TNM staging system provides an incomplete biologic profile of NSCLC, does not always provide a satisfactory explanation for differences in relapse and survival and is thus far from perfect as a prognostic indicator[12]. Quantitative measures of biological aggressiveness, like FDG uptake, seem to be better indicators for survival and risk of relapse[12–15]. For prognostic stratification a semi-quantitative value that expresses glucose use (i.e. the standardized uptake value or SUV) can be calculated using a single whole body FDG-PET that is routinely performed as part of the pre-therapeutic staging procedure. A great advantage of measurement of FDG uptake is that this can be done before any treatment has been performed.
The prognostic value of FDG-PET at diagnosis12,[15–24], after induction therapy[25–28] or in recurrent disease[29],[30] has been evaluated. These studies have shown that pre-therapeutic FDG-PET not only improved patient staging, but also provided prognostic information. Patients with low FDG uptake in their primary tumour have a significant longer overall and progression-free survival than patients with high FDG uptake. Several studies[16],[17],[21] found that in patients with high FDG uptake, prognosis was further reduced if the tumour also exceeded 3 cm in size.
In contrast to FDG uptake in the primary tumour, the prognostic ability of the SUV for the regional lymph nodes remains uncertain. Sasaki et al.[15] observed that patients with high SUVs of their regional lymph nodes and low SUVs in their primary tumours did not experience any local or distant relapse. Therefore, it is at least speculated that the SUVs for the regional lymph nodes do not agree with and are not stronger prognostic factors than the SUVs for the primary tumour. Patients with complete resolution of prior FDG positive lesions have been shown to have a good prognosis compared to those with residual FDG uptake after induction treatment.
Univariate analyses performed to determine a cut-off point for the SUV in the primary tumour to discriminate between a more or less favourable prognosis has ranged widely from 5 to 20. Vansteenkiste and Higashi et al.[12],[17] showed that dichotomization with a broad range of SUVs gave significantly discriminative log-rank probability values. This implies that the relationship between SUV and prognosis could be a gradual one rather than based on a threshold. It seems reasonable to hypothesize that there is no true cut-off point but, rather, a transition zone, within which the prognosis gradually worsens.
However, the wide range of SUV values seen in these studies can also be due to the heterogeneity of the patient cohorts analysed and to variation in the PET scanners and acquisition protocols used. Institutional-based technical factors can lead to variations in measurement of SUV and might hinder the integrated or comparative interpretation of the results from one centre to another[31],[32]. Therefore, standardization of acquisition, reconstruction and ROI methods is preferred for SUV quantification in multi-centre trials[32]. Agreement on methods of scanning, SUV measurement and the best cut-off values is needed before this technique can be fully exploited in clinical medicine to select patients for adjuvant treatments. Nevertheless, despite these variabilities and concerns, all 17 studies arrived at the same conclusion and strongly confirmed that the degree of tumour glucose use on an FDG-PET scan provides independent prognostic information.
Predictive value of FDG-PET in NSCLC
Although the hallmark for evaluation of therapeutic effectiveness of cancer treatment, current morphological imaging techniques have limitations in reliably distinguishing necrotic tumour or fibrotic scar from residual tumour tissue[26]. Metabolic response correlated better to pathologic response than the change in size on CT and proved to be a better predictor of long-term survival[33–38]. Indeed, final treatment outcome will be determined more by the biological aggressiveness of residual tumour than by its volume[39].
A reduction in glucose use by tumour cells, indicative of tumour response to therapy, may occur before alterations in tumour size. There seems to be a near linear relationship between the change in tumour glucose use (as expressed in the SUV) and the percent of non-viable tumour cells in resected tumours[36],[37]. Fourteen studies indicated a possible role for PET in assessment of response during chemotherapy[40–43], induction chemotherapy[28],[44] during or after radiotherapy[45],[46], or a combination thereof26,[35–38],47. There are significantly more complete responders to chemoradiation on PET than CT, whereas fewer patients are judged to be non-responders, which implies that chemoradiation may be a more effective therapy than previous CT-based assessments have suggested[26]. Based on the residual metabolic rate of glucose after one cycle of chemotherapy, patients with different outcomes can be selected[28],[40].
Early prediction of tumour response is of particular interest in patients with advanced stages of NSCLC. Tumour progression during first-line chemotherapy occurs in approximately 30% of patients[48]. Due to relatively slow tumour shrinkage as measured on morphological imaging modalities, a significant percentage of patients will continue to undergo toxic therapy for weeks without benefit. At present, there are no systematic data available on patients with NSCLC to determine the optimal time to perform FDG-PET after radiotherapy. However, the influence of radiation induced inflammatory reactions does not seem a major issue since all the reports in a post-radiotherapy setting did not show major confounding results26,[28],[35],[38],45,47,[49–52]. Despite the fact that the 12 studies on therapy response assessment were very heterogeneous with respect to the methods of PET quantification and data analysis applied, the primary targets of PET evaluation (primary tumour and/or lymph nodes), tumour stage, tumour type, and the clinical end points (histology, survival), all studies showed that FDG-PET is a significant predictor of therapy outcome and provides results of great prognostic significance (Fig. 1).
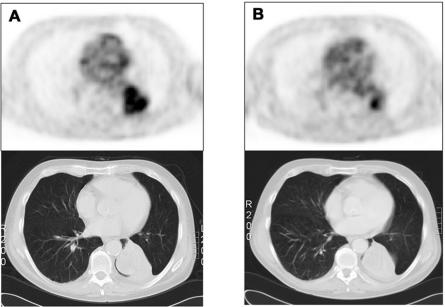
Figure 1.
Typical example of a patient with stage IV NSCLC and tumour response to chemotherapy. Relative to baseline (A), there is an obvious metabolic response on FDG-PET after two cycles of carboplatin/gemcitabine (B). The lung lesion in combination with some atelectasis persists indefinitely on the post-therapy (B) CT scan, which cannot distinguish residual vital tumour.
Prognostic and predictive value of FDG-PET in colorectal carcinoma
FDG-PET has an established role in staging patients before surgical resection of recurrence and metastases, in the localization of recurrence in patients with an unexplained rise of serum carcinoembryonic antigen, and distinguishing fibrosis and scar from viable tumour in residual masses of rectal cancer after treatment[53]. In the pre-surgical evaluation, FDG-PET may be best used in conjunction with anatomic imaging in order to combine the benefits of both anatomical (CT) and functional (PET) information, which leads to significant improvements in pre-operative liver staging and pre-operative judgement on the feasibility of resection. Integration of FDG-PET into the management algorithm of these categories of patients alters and improves therapeutic management, reduces morbidity due to futile surgery, leads to substantial cost savings and probably also to a better patient outcome[54],[55]. The following paragraph presents a literature review of its emerging role in the prediction and evaluation of treatment response, such as monitoring of radiotherapy and multimodality treatment response in primary rectal cancer[56–62], monitoring response after local ablative therapy of liver metastases[63–66] and monitoring chemotherapy response in advanced colorectal cancer[67–71].
Several investigators have speculated that the amount of FDG uptake correlates with biologic factors such as Ki-67, proliferating cell nuclear antigen, Glut-1, and hexokinase[72] and that FDG uptake resembles the biological behaviour of the tumour, and might be associated with intrinsic biologic characteristics, like hypoxia[73], low apoptosis rate[74], cell viability[75], proliferative activity[76] and p53 overexpression[77]. These characteristics are all potentially adverse factors in patients treated with radiotherapy or chemotherapy, while some of them may also impact negatively in patients treated surgically. To the best of our knowledge, there are only two studies[78],[79] addressing the prognostic value of FDG-PET in patients with metastatic colorectal carcinoma. Pre-treatment FDG uptake in metastatic colorectal cancer predicted outcome, irrespective of the subsequent treatment modality, as patients with FDG avid disease showed reduced overall survival.
In rectal cancer, pre-operative chemoradiotherapy is used in advanced T3 and T4 tumours in an attempt to down-stage the disease, in order to reduce the risk of local recurrence and to allow sphincter preserving tumour resection in selected cases[80],[81]. FDG-PET may have a role in pre-operative multimodality treatment response evaluation and in a pre-operative strategy aimed at identifying patients most suitable for sphincter preserving surgery[56],[58]. Reduction in SUV was significantly greater in (histopathologically confirmed) responders compared to non-responders and predicted therapy outcome significantly better than endorectal ultrasound, CT and magnetic resonance imaging (MRI)56,[57],[59],[60].
It is striking that the confounding radiotherapy-induced effects, as discussed earlier, have less impact on the results of FDG-PET if it is combined with chemotherapy and/or regional hyperthermia. This implies that the nature of the combination of treatment modalities for neoadjuvant therapy is important in the timing of FDG-PET evaluation. Further studies are required to ascertain the exact sequence of time-dependent (radio)biological effects during neoadjuvant multimodality treatment. For induction radiotherapy alone, it has not yet been sufficiently investigated whether FDG-PET could play a role in the pre-operative radiotherapy response assessment of primary rectal cancer. The generally accepted interval of at least 6 months for FDG-PET evaluation after adjuvant radiotherapy is not applicable in a neoadjuvant setting. Probably due to these expected confounding radiotherapy-induced effects on FDG uptake, only one study on this subject has been performed[61]. They found that an overall decrease of glucose utilization correlated to reduction of tumour burden and cell death and was predictive for therapy outcome as early as 2 weeks after radiotherapy. These surprising results call for systematic investigation of the required interval for post-radiotherapy evaluation with FDG-PET.
For patients with colorectal liver metastases, surgical resection offers the best chances for cure. In most patients with colorectal liver metastases, however, resection cannot be performed. When this is caused by the number and/or localization of metastases, local ablative techniques such as cryosurgery or radiofrequency ablation may offer an alternative treatment that produces localized intrahepatic tumour destruction and possibly results in a prolongation of survival. A prospective randomized trial on the impact of radiofrequency ablation versus chemotherapy (CLOCC) is on-going.
Different morphological imaging techniques have been used to facilitate intra-operative localization. However, during the process of local ablation the destruction process cannot easily be ascertained with intra-operative ultrasound imaging because of the hyperechogenicity that is induced within the treated area[82]. Furthermore, evaluation with CT scanning or MRI of residual tumour after the ablation procedure is limited because post-treatment hyperaemia or tissue regeneration may result in contrast enhancement in the periphery of the ablative necrosis[83]. This can lead to either a delayed diagnosis of treatment failure or to confusion between incomplete local ablative treatment and the occurrence of new metastases in regions adjacent to the treatment site. Several studies have described the feasibility of FDG-PET scanning in the surveillance of these patients[63–66]. It appears to have great potential in identifying residual tumour soon after local ablative treatments. The negative predictive value of FDG-PET at 3 months was 100%. The data presented indicate that FDG-PET could play a central role in optimizing the use of local ablative treatment of liver metastases as it recognizes early incomplete tumour ablation that is not detectable by CT scanning. FDG-PET determines the need for further investigations and guides the reading of the CT scan, which on its own appears difficult to interpret in the early period after local ablative therapy. The combined information of FDG-PET and CT scans offers the opportunity to re-treat tumours at an early stage.
There are five reports suggesting that FDG-PET can predict response to chemotherapy in patients with irresectable colorectal cancer liver metastases[67–71] (Fig. 2). A clear correlation was observed between the reduction of tumour metabolism 5 weeks after the initiation of chemotherapy and treatment outcome, which was not observed at 1–2 weeks on treatment[67]. These results show the importance of the correct timing of FDG-PET after the onset of chemotherapy. The authors mention the so-called flare phenomenon that occurs 1–2 weeks after the initiation of chemotherapy, which can be observed as a marked increase in FDG metabolism in lesions that show a response later on. Bender et al.[68] showed that the flare phenomenon probably does not play a role as early as 72 h after initiation of chemotherapy. These preliminary data indicate that acute changes of glucose utilization following a single application of chemotherapy seem to be indicative for the final therapeutic outcome.
Figure 2.
Transversal slice through the liver at baseline (A) and after 2 months of chemotherapy (B) in a patient with liver metastases of colorectal cancer. After 2 months of chemotherapy there is a complete metabolic response; MRI still shows a liver lesion of 7 cm in diameter.
Predictive and prognostic value of FDG-PET in lymphoma
In the treatment of curable lymphomas (e.g. Hodgkin's lymphoma (HL) and aggressive, i.e. high-grade non-Hodgkin's lymphoma (NHL) or diffuse large B-cell lymphoma (DLBCL)), the goal of treatment is to achieve a complete response, which is a prerequisite for cure[84]. Patients who do not achieve a complete response by the end of treatment are offered extra or salvage treatment. CT is often unable to differentiate between viable tumour, necrosis, or fibrosis in a residual mass (Fig. 3). The awareness of long-term toxicities and treatment related disease, especially after chemoradiation, has questioned the standard use of radiotherapy on a residual mass[85]. The introduction of patient tailored therapy raised the interest for accurate assessment of response. Recently, FDG-PET has been introduced for post-treatment remission assessment of these FDG-avid, potentially curable lymphomas. A high negative predictive value of FDG-PET is consistently reported in DLBCL and HL, and a negative FDG-PET clearly identifies patients with an excellent prognosis[86]. Classical HL deserves special consideration in this regard. In patients with HL the significance of a positive FDG-PET scan is less clear, since typically less than 1% of the tumour mass comprises malignant cells; the remainder is a benign inflammatory infiltrate[87]. In general, a negative scan in HL is indicative of a good prognosis, whereas a positive scan should be interpreted in concert with other staging investigations. in contrast, in histologically aggressive NHL, there appear to be few false positive results but negative FDG-PET scans must be viewed with some caution[88]. CT-defined FDG-PET negative residual masses require no further treatment. FDG-PET-positive residual masses require further treatment, preferably after histological confirmation. The prognostic value of FDG-PET for curable lymphomas has been established, however, the next step is to define how to use this information to optimize patient outcomes. The role of metabolic response assessment in aggressive NHL subtypes other than DLBCL, and in indolent and mantle-cell lymphomas, is questionable. Most low-grade lymphomas relapse over time, regardless of the initial response obtained and early salvage therapy has not yet been proven to change survival.
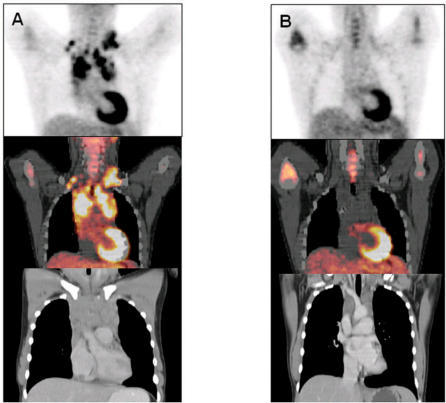
Figure 3.
(A) Pre-treatment and (B) post-treatment PET-CT of a patient with a stage IIA nodular sclerosing Hodgkin's lymphoma. The baseline FDG-PET shows advanced FDG avid disease. A first follow-up study shows complete metabolic response, whereas the CT scan shows residual mediastinal abnormalities. The patient was classified as having CRu (complete response unconfirmed) by International Workshop Criteria (IWC) and complete response by IWC + PET.
Although HL, DLBCL, follicular lymphoma and mantle-cell lymphoma are routinely FDG avid, pre-therapy FDG-PET for assessment of response after treatment is strongly encouraged, because it can facilitate the interpretation of post-therapy FDG-PET[89–91]. However, pre-treatment FDG-PET is obligatory for variably FDG avid lymphomas, like aggressive NHL subtypes other than DLBCL, such as T-cell lymphomas, and all subtypes of indolent NHL other than follicular lymphoma, such as extranodal marginal zone lymphoma of mucosa associated lymphoid tissue (MALT) and small lymphocytic lymphoma, to document pre-treatment FDG avidity at all disease sites noted by CT[89],[92],[93].
The imaging subcommittee of the International Harmonization Project (IHP) recommended metabolic response assessment at the conclusion of therapy at least 3 weeks after chemo(immuno)therapy and preferably 8–12 weeks after completion of (chemo)radiation, in order to minimize false-positive interpretation, due to transient inflammatory changes as a reaction to therapy[91]. As benign inflammatory FDG uptake in a residual mass rarely exceeds FDG uptake in the mediastinum, a comparison between uptake in the residual mass and the mediastinum has been proposed by the IHP as a reference cut-off for standardization of PET criteria. Residual masses, regardless of their location, with uptake less than or equal to mediastinal activity should be considered negative for lymphoma, while uptake greater than mediastinal uptake should be considered as residual disease. However, as FDG-uptake in a residual mass less than 2 cm in diameter is artificially decreased because of the effect of partial volume averaging, any uptake higher than the surrounding background should be suggestive for residual lymphoma. Where abnormal FDG uptake is seen outside the sites involved initially, infectious/inflammatory lesions and thymic hyperplasia have first to be excluded[94]. Such lesions should only be considered relapsed or progressive disease after confirmation with other modalities.
Specific criteria for lung nodules have been developed. New lung nodules >1.5 cm as measured by CT in a patient with no evidence of pulmonary lymphoma before therapy should only be considered suggestive of lymphoma if nodular uptake exceeds that of the mediastinum and should always be confirmed with other modalities and ultimately need histologic confirmation. In cases of complete response at all previously known disease sites, these new lung FDG-avid nodules should be considered negative for lymphoma regardless of their size or uptake because these typically represent infectious or inflammatory lesions. Unfortunately, residual lymphoma cannot be excluded in pre-existent small nodules <1.5 cm in size, that fail to show FDG uptake[91].
FDG uptake that exceeds liver or spleen uptake in pre-existing hepatic or splenic lesions that are >1.5 cm as measured on CT should be considered positive for lymphoma. If their uptake is less than that of the liver or spleen they should be considered negative for lymphoma, whereas persistent hepatic/splenic lesions <1.5 cm in diameter should be considered negative if their uptake is equal to or lower than surrounding liver or spleen uptake. Recent cytokine administration can cause diffusely increased splenic uptake for up to 10 days after cessation of cytokine administration[95]. If the patient has no history of cytokine administration, diffusely increased splenic uptake greater than normal liver uptake should be interpreted as positive for lymphoma.
Diffusely increased bone marrow uptake, is usually due to post-therapy marrow hyperplasia. However, obviously increased (multi)focal bone marrow uptake should be considered positive for lymphoma. Bone marrow biopsy, however, remains the standard procedure for assessment of bone marrow, since a negative FDG uptake in the bone marrow does not exclude mild or moderate bone marrow involvement[96],[97].
In addition to the standardization of FDG-PET interpretation, the IHP has formulated guidelines for integration of FDG-PET and CT results (IWC + PET criteria, see Table 1)[98],[99]. In the original International Workshop Criteria (IWC), based on CT response assessment, complete response unconfirmed (CRu) was defined by complete disappearance of all detectable clinical evidence of disease, normalization of biochemical abnormalities and disease-related symptoms, but with a residual mass more than 1.5 cm in diameter that has regressed by more than 75% or indeterminate bone marrow (increased number or size of aggregates without cytologic or architectural atypia). FDG-PET increased the number of patients with complete remission by eliminating the CRu category, and enhanced the ability to discern the difference in progression-free survival between patients with complete and partial remission (Fig. 3). It is important that these guidelines are adopted widely by study groups, pharmaceutical and biotechnology companies, and regulatory agencies to facilitate the development of new and more effective therapies to improve the outcome of patients with lymphoma.
Table 1.
| IWC + PET response designations | Description |
|---|---|
| CR | CR, CRu, PR or SD by IWC; PET completely negative; BMB negative if positive prior to therapy |
| PR | CR, CRu, PR by IWC; PET positive |
| SD | SD by IWC; PET positive |
| PD | PD by IWC; PET positive corresponding to the CT abnormality |
IWC + PET, International Workshop Criteria and positron emission tomography; CR, complete remission; BMB, bone marrow biopsy; CT, computed tomography; CRu, unconfirmed CR; PR, partial response; SD, stable disease; PD, progressive disease.
Use of FDG-PET for early response assessment during a course of therapy should only be done in a clinical trial or as part of a prospective registry. Response-adapted treatment aims to optimize the balance between cure and toxicity for the individual patient. Early response assessment could enable this strategy in the future by minimizing treatment for patients with a good prognosis and intensifying treatment for patients with a poor prognosis. Most authors are convinced that interim FDG-PET has at least an equally strong prognostic value compared to end-of-treatment FDG-PET, but the optimal timing of interim PET is not yet clear. Several studies have demonstrated the predictive value of FDG-PET as early as after one to four cycles of chemo(immuno)therapy, although no treatment change based on early PET results has proven to alter patients outcome yet[100],[101]. During a course of therapy, FDG-PET should be performed as close as possible before the subsequent cycle[91]. It is not yet clear whether visual or (semi)quantitative assessment is sufficiently reliable to distinguish patients with a more favourable from those with less favourable outcome. Nevertheless, measurements of fractional changes in glucose use over time are only reliable when sequential scans of the same patient are performed using the same scanner, under identical scanning, image reconstruction and data analysis conditions, what means that serial scans of one patient should be performed in the same institute. A clear cut-off for an adequate (clinically meaningful) reduction in glucose use remains to be defined in large trials and may vary on the basis of tumour histology and type of treatment.
Final remarks
This review provides an overview of the literature on the prognostic value of FDG-PET at initial presentation of NSCLC and metastatic colorectal carcinoma, after induction treatment of NSCLC and lymphoma and in the case of relapse of NSCLC. In other malignancies, such as head and neck squamous cell carcinoma[102–108], breast cancer[77],[109],[110], glioma[111], oesophageal carcinoma[112],[113], pancreatic cancer[114–117] and hepatocellular carcinoma[118] FDG-PET also proved to be an independent prognostic marker. As was reported in gastrointestinal stromal tumours[119],[120], oesophageal carcinoma[121–123], gastric carcinoma[124], head and neck squamous cell carcinoma[125] and cervix carcinoma[126], FDG-PET proved to have a predictive value early in the course of treatment of NSCLC and lymphoma. In colorectal carcinoma, FDG-PET also appears to have great potential in monitoring the success of local ablative therapies soon after intervention and in the evaluation of response to radiotherapy, systemic therapy, and combinations thereof. Further research in this field is of great importance, since it may induce a change in the therapeutic concept of oncological patients. If the results of the reviewed studies can be confirmed, FDG-PET could shorten the track of early clinical trials that assess new anti-neoplastic agents and could also improve patient management by reducing morbidity, efforts and costs of ineffective treatment in non-responders.
References
- [1].Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–24. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- [2].Bury T, Dowlati A, Paulus P, et al. Evaluation of the solitary pulmonary nodule by positron emission tomography imaging. Eur Respir J. 1996;9:410–4. doi: 10.1183/09031936.96.09030410. [DOI] [PubMed] [Google Scholar]
- [3].Verhagen AF, Bootsma GP, Tjan-Heijnen VC, et al. FDG-PET in staging lung cancer: how does it change the algorithm? Lung Cancer. 2004;44:175–81. doi: 10.1016/j.lungcan.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [4].van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–93. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- [5].Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- [6].Vesselle H, Turcotte E, Wiens L, et al. Relationship between non-small cell lung cancer fluorodeoxyglucose uptake at positron emission tomography and surgical stage with relevance to patient prognosis. Clin Cancer Res. 2004;10:4709–16. doi: 10.1158/1078-0432.CCR-03-0773. [DOI] [PubMed] [Google Scholar]
- [7].Ashamalla H, Rafla S, Parikh K, et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:1016–23. doi: 10.1016/j.ijrobp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- [8].Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354:496–507. doi: 10.1056/NEJMra050276. [DOI] [PubMed] [Google Scholar]
- [9].De Ruysscher D, Wanders S, van Haren E, et al. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non-small-cell lung cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys. 2005;62:988–94. doi: 10.1016/j.ijrobp.2004.12.019. [DOI] [PubMed] [Google Scholar]
- [10].van Der Wel A, Nijsten S, Hochstenbag M, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys. 2005;61:649–55. doi: 10.1016/j.ijrobp.2004.06.205. [DOI] [PubMed] [Google Scholar]
- [11].van Rens MT, de la Riviere AB, Elbers HR, van den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117:374–9. doi: 10.1378/chest.117.2.374. [DOI] [PubMed] [Google Scholar]
- [12].Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- [13].Higashi K, Ito K, Hiramatsu Y, et al. 18F-FDG uptake by primary tumor as a predictor of intratumoral lymphatic vessel invasion and lymph node involvement in non-small cell lung cancer: analysis of a multicenter study. J Nucl Med. 2005;46:267–73. [PubMed] [Google Scholar]
- [14].Higashi K, Ueda Y, Ayabe K, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun. 2000;21:707–14. doi: 10.1097/00006231-200008000-00002. [DOI] [PubMed] [Google Scholar]
- [15].Sasaki R, Komaki R, Macapinlac H, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005;23:1136–43. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- [16].Ahuja V, Coleman RE, Herndon J, Patz EF., Jr The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer. 1998;83:918–24. [PubMed] [Google Scholar]
- [17].Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on 18F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999;17:3201–6. doi: 10.1200/JCO.1999.17.10.3201. [DOI] [PubMed] [Google Scholar]
- [18].Sugawara Y, Quint LE, Iannettoni MD, et al. Does the FDG uptake of primary non-small cell lung cancer predict prognosis? A work in progress. Clin Positron Imaging. 1999;2:111–8. doi: 10.1016/s1095-0397(99)00012-6. [DOI] [PubMed] [Google Scholar]
- [19].Dhital K, Saunders CA, Seed PT, O’Doherty MJ, Dussek J. [18F]Fluorodeoxyglucose positron emission tomography and its prognostic value in lung cancer. Eur J Cardiothorac Surg. 2000;18:425–8. doi: 10.1016/s1010-7940(00)00535-2. [DOI] [PubMed] [Google Scholar]
- [20].Jeong HJ, Min JJ, Park JM, et al. Determination of the prognostic value of [18F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun. 2002;23:865–70. doi: 10.1097/00006231-200209000-00010. [DOI] [PubMed] [Google Scholar]
- [21].Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–60. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- [22].Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005;130:151–9. doi: 10.1016/j.jtcvs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [23].Borst GR, Belderbos JS, Boellaard R, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer. 2005;41:1533–41. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [24].Eschmann SM, Friedel G, Paulsen F, et al. Is standardised 18F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging. 2006;33:263–9. doi: 10.1007/s00259-005-1953-2. [DOI] [PubMed] [Google Scholar]
- [25].Patz EF, Jr, Connolly J, Herndon J. Prognostic value of thoracic FDG PET imaging after treatment for non-small cell lung cancer. AJR Am J Roentgenol. 2000;174:769–74. doi: 10.2214/ajr.174.3.1740769. [DOI] [PubMed] [Google Scholar]
- [26].Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- [27].Hellwig D, Graeter TP, Ukena D, et al. Value of F-18-fluorodeoxyglucose positron emission tomography after induction therapy of locally advanced bronchogenic carcinoma. J Thorac Cardiovasc Surg. 2004;128:892–9. doi: 10.1016/j.jtcvs.2004.07.031. [DOI] [PubMed] [Google Scholar]
- [28].Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-d-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362–70. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- [29].Hellwig D, Groschel A, Graeter TP, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:13–21. doi: 10.1007/s00259-005-1919-4. [DOI] [PubMed] [Google Scholar]
- [30].Hicks RJ, Kalff V, MacManus MP, et al. The utility of 18F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42:1605–13. [PubMed] [Google Scholar]
- [31].Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27. [PubMed] [Google Scholar]
- [32].Westerterp M, Pruim J, Oyen W, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2007;34:392–404. doi: 10.1007/s00259-006-0224-1. [DOI] [PubMed] [Google Scholar]
- [33].Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [34].Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–53. doi: 10.1007/BF00944177. [DOI] [PubMed] [Google Scholar]
- [35].Choi NC, Fischman AJ, Niemierko A, et al. Dose–response relationship between probability of pathologic tumor control and glucose metabolic rate measured with FDG PET after preoperative chemoradiotherapy in locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:1024–35. doi: 10.1016/s0360-3016(02)03038-9. [DOI] [PubMed] [Google Scholar]
- [36].Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78:1903–9. doi: 10.1016/j.athoracsur.2004.06.102. [DOI] [PubMed] [Google Scholar]
- [37].Pottgen C, Levegrun S, Theegarten D, et al. Value of 18F-fluoro-2-deoxy-d-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res. 2006;12:97–106. doi: 10.1158/1078-0432.CCR-05-0510. [DOI] [PubMed] [Google Scholar]
- [38].Yamamoto Y, Nishiyama Y, Monden T, et al. Correlation of FDG-PET findings with histopathology in the assessment of response to induction chemoradiotherapy in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:140–7. doi: 10.1007/s00259-005-1878-9. [DOI] [PubMed] [Google Scholar]
- [39].Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–40. doi: 10.1016/S1470-2045(04)01564-5. [DOI] [PubMed] [Google Scholar]
- [40].Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [41].Lee KH, Lee SH, Kim DW, et al. High fluorodeoxyglucose uptake on positron emission tomography in patients with advanced non-small cell lung cancer on platinum-based combination chemotherapy. Clin Cancer Res. 2006;12:4232–6. doi: 10.1158/1078-0432.CCR-05-2710. [DOI] [PubMed] [Google Scholar]
- [42].de Geus-Oei LF, van der Heijden HF, Visser EP, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–8. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- [43].Nahmias C, Hanna WT, Wahl LM, et al. Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med. 2007;48:744–51. doi: 10.2967/jnumed.106.038513. [DOI] [PubMed] [Google Scholar]
- [44].Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Verbeken EK. Potential use of FDG-PET scan after induction chemotherapy in surgically staged IIIa-N2 non-small-cell lung cancer: a prospective pilot study. The Leuven Lung Cancer Group. Ann Oncol. 1998;9:1193–8. doi: 10.1023/a:1008437915860. [DOI] [PubMed] [Google Scholar]
- [45].Ichiya Y, Kuwabara Y, Sasaki M, et al. A clinical evaluation of FDG-PET to assess the response in radiation therapy for bronchogenic carcinoma. Ann Nucl Med. 1996;10:193–200. doi: 10.1007/BF03165392. [DOI] [PubMed] [Google Scholar]
- [46].Kong FM, Frey KA, Quint LE, et al. A pilot study of [18F]fluorodeoxyglucose positron emission tomography scans during and after radiation-based therapy in patients with non small-cell lung cancer. J Clin Oncol. 2007;25:3116–23. doi: 10.1200/JCO.2006.10.3747. [DOI] [PubMed] [Google Scholar]
- [47].Mac Manus MP, Hicks RJ, Matthews JP, et al. Metabolic (FDG-PET) response after radical radiotherapy/chemoradiotherapy for non-small cell lung cancer correlates with patterns of failure. Lung Cancer. 2005;49:95–108. doi: 10.1016/j.lungcan.2004.11.024. [DOI] [PubMed] [Google Scholar]
- [48].Sekine I, Tamura T, Kunitoh H, et al. Progressive disease rate as a surrogate endpoint of phase II trials for non-small-cell lung cancer. Ann Oncol. 1999;10:731–3. doi: 10.1023/a:1008303921033. [DOI] [PubMed] [Google Scholar]
- [49].Abe Y, Matsuzawa T, Fujiwara T, et al. Clinical assessment of therapeutic effects on cancer using 18F-2-fluoro-2-deoxy-d-glucose and positron emission tomography: preliminary study of lung cancer. Int J Radiat Oncol Biol Phys. 1990;19:1005–10. doi: 10.1016/0360-3016(90)90026-g. [DOI] [PubMed] [Google Scholar]
- [50].Erdi YE, Macapinlac H, Rosenzweig KE, et al. Use of PET to monitor the response of lung cancer to radiation treatment. Eur J Nucl Med. 2000;27:861–6. doi: 10.1007/s002590000258. [DOI] [PubMed] [Google Scholar]
- [51].Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–87. doi: 10.1016/s0169-5002(01)00332-4. [DOI] [PubMed] [Google Scholar]
- [52].Hebert ME, Lowe VJ, Hoffman JM, Patz EF, Anscher MS. Positron emission tomography in the pretreatment evaluation and follow-up of non-small cell lung cancer patients treated with radiotherapy: preliminary findings. Am J Clin Oncol. 1996;19:416–21. doi: 10.1097/00000421-199608000-00020. [DOI] [PubMed] [Google Scholar]
- [53].de Geus-Oei LF, Ruers TJ, Punt CJ, et al. FDG-PET in colorectal cancer. Cancer Imaging. 2006;6:S71–S81. doi: 10.1102/1470-7330.2006.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ruers TJ, Langenhoff BS, Neeleman N, et al. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388–95. doi: 10.1200/JCO.2002.20.2.388. [DOI] [PubMed] [Google Scholar]
- [55].Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104:2658–70. doi: 10.1002/cncr.21569. [DOI] [PubMed] [Google Scholar]
- [56].Amthauer H, Denecke T, Rau B, et al. Response prediction by FDG-PET after neoadjuvant radiochemotherapy and combined regional hyperthermia of rectal cancer: correlation with endorectal ultrasound and histopathology. Eur J Nucl Med Mol Imaging. 2004;31:811–9. doi: 10.1007/s00259-003-1453-1. [DOI] [PubMed] [Google Scholar]
- [57].Denecke T, Rau B, Hoffmann KT, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15:1658–66. doi: 10.1007/s00330-005-2658-4. [DOI] [PubMed] [Google Scholar]
- [58].Guillem JG, Puig-La CJ, Jr, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000;43:18–24. doi: 10.1007/BF02237238. [DOI] [PubMed] [Google Scholar]
- [59].Guillem JG, Moore HG, Akhurst T, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004;199:1–7. doi: 10.1016/j.jamcollsurg.2004.02.024. [DOI] [PubMed] [Google Scholar]
- [60].Calvo FA, Domper M, Matute R, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58:528–35. doi: 10.1016/j.ijrobp.2003.09.058. [DOI] [PubMed] [Google Scholar]
- [61].Schiepers C, Haustermans K, Geboes K, et al. The effect of preoperative radiation therapy on glucose utilization and cell kinetics in patients with primary rectal carcinoma. Cancer. 1999;85:803–11. doi: 10.1002/(sici)1097-0142(19990215)85:4<803::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [62].Cascini GL, Avallone A, Delrio P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47:1241–8. [PubMed] [Google Scholar]
- [63].Langenhoff BS, Oyen WJ, Jager GJ, et al. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453–8. doi: 10.1200/JCO.2002.12.134. [DOI] [PubMed] [Google Scholar]
- [64].Joosten J, Jager G, Oyen W, Wobbes T, Ruers T. Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. Eur J Surg Oncol. 2005;31:1152–9. doi: 10.1016/j.ejso.2005.07.010. [DOI] [PubMed] [Google Scholar]
- [65].Donckier V, Van Laethem JL, Goldman S, et al. [F-18]Fluorodeoxyglucose positron emission tomography as a tool for early recognition of incomplete tumor destruction after radiofrequency ablation for liver metastases. J Surg Oncol. 2003;84:215–23. doi: 10.1002/jso.10314. [DOI] [PubMed] [Google Scholar]
- [66].Blokhuis TJ, van der Schaaf MC, van den Tol MP, et al. Results of radio frequency ablation of primary and secondary liver tumors: long-term follow-up with computed tomography and positron emission tomography-18F-deoxyfluoroglucose scanning. Scand J Gastroenterol Suppl. 2004:93–7. doi: 10.1080/00855920410014623. [DOI] [PubMed] [Google Scholar]
- [67].Findlay M, Young H, Cunningham D, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol. 1996;14:700–8. doi: 10.1200/JCO.1996.14.3.700. [DOI] [PubMed] [Google Scholar]
- [68].Bender H, Bangard N, Metten N, et al. Possible role of FDG-PET in the early prediction of therapy outcome in liver metastases of colorectal cancer. Hybridoma. 1999;18:87–91. doi: 10.1089/hyb.1999.18.87. [DOI] [PubMed] [Google Scholar]
- [69].Dimitrakopoulou-Strauss A, Strauss LG, Rudi J. PET-FDG as predictor of therapy response in patients with colorectal carcinoma. Q J Nucl Med. 2003;47:8–13. [PubMed] [Google Scholar]
- [70].Dimitrakopoulou-Strauss A, Strauss LG, Burger C, et al. Prognostic aspects of 18F-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med. 2004;45:1480–7. [PubMed] [Google Scholar]
- [71].de Geus-Oei LF, van Laarhoven HW, Visser EP, et al. Chemotherapy response evaluation with FDG PET in patients with colorectal cancer. Ann Oncol. 2008;19:348–52. doi: 10.1093/annonc/mdm470. [DOI] [PubMed] [Google Scholar]
- [72].Akhurst T, Kates TJ, Mazumdar M, et al. Recent chemotherapy reduces the sensitivity of [18F]fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol. 2005;23:8713–6. doi: 10.1200/JCO.2005.04.4222. [DOI] [PubMed] [Google Scholar]
- [73].Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625–32. [PubMed] [Google Scholar]
- [74].Furuta M, Hasegawa M, Hayakawa K, et al. Rapid rise in FDG uptake in an irradiated human tumour xenograft. Eur J Nucl Med. 1997;24:435–8. doi: 10.1007/BF00881817. [DOI] [PubMed] [Google Scholar]
- [75].Minn H, Clavo AC, Grenman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose and L-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med. 1995;36:252–8. [PubMed] [Google Scholar]
- [76].Haberkorn U, Strauss LG, Reisser C, et al. Glucose uptake, perfusion, and cell proliferation in head and neck tumors: relation of positron emission tomography to flow cytometry. J Nucl Med. 1991;32:1548–55. [PubMed] [Google Scholar]
- [77].Crippa F, Seregni E, Agresti R, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25:1429–34. doi: 10.1007/s002590050319. [DOI] [PubMed] [Google Scholar]
- [78].de Geus-Oei LF, Wiering B, Krabbe PF, et al. FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol. 2006;17:1650–5. doi: 10.1093/annonc/mdl180. [DOI] [PubMed] [Google Scholar]
- [79].Riedl CC, Akhurst T, Larson S, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48:771–5. doi: 10.2967/jnumed.106.037291. [DOI] [PubMed] [Google Scholar]
- [80].Losi L, Luppi G, Gavioli M, et al. Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis. 2005:1–7. doi: 10.1007/s00384-005-0061-x. [DOI] [PubMed] [Google Scholar]
- [81].Hughes R, Glynne-Jones R, Grainger J, et al. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis. 2006;21:11–7. doi: 10.1007/s00384-005-0749-y. [DOI] [PubMed] [Google Scholar]
- [82].Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–22. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- [83].Antoch G, Vogt FM, Veit P, et al. Assessment of liver tissue after radiofrequency ablation: findings with different imaging procedures. J Nucl Med. 2005;46:520–5. [PubMed] [Google Scholar]
- [84].Mikhaeel NG. Use of FDG-PET to monitor response to chemotherapy and radiotherapy in patients with lymphomas. Eur J Nucl Med Mol Imaging. 2006;33(Suppl 1):22–6. doi: 10.1007/s00259-006-0132-4. [DOI] [PubMed] [Google Scholar]
- [85].Brepoels L, Stroobants S, Verhoef G. PET and PET/CT for response evaluation in lymphoma: current practice and developments. Leuk Lymphoma. 2007;48:270–82. doi: 10.1080/10428190601078118. [DOI] [PubMed] [Google Scholar]
- [86].Kirby AM, Mikhaeel NG. The role of FDG PET in the management of lymphoma: what is the evidence base? Nucl Med Commun. 2007;28:335–54. doi: 10.1097/MNM.0b013e3280895e23. [DOI] [PubMed] [Google Scholar]
- [87].Kasamon YL, Jones RJ, Wahl RL. Integrating PET and PET/CT into the risk-adapted therapy of lymphoma. J Nucl Med. 2007;48(Suppl 1):19S–27S. [PubMed] [Google Scholar]
- [88].Zinzani PL, Magagnoli M, Chierichetti F, et al. The role of positron emission tomography (PET) in the management of lymphoma patients. Ann Oncol. 1999;10:1181–4. doi: 10.1023/a:1008327127033. [DOI] [PubMed] [Google Scholar]
- [89].Elstrom R, Guan L, Baker G, et al. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875–6. doi: 10.1182/blood-2002-09-2778. [DOI] [PubMed] [Google Scholar]
- [90].Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:4643–51. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- [91].Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- [92].Hoffmann M, Kletter K, Diemling M, et al. Positron emission tomography with fluorine-18-2-fluoro-2-deoxy-d-glucose (F18-FDG) does not visualize extranodal B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT)-type. Ann Oncol. 1999;10:1185–9. doi: 10.1023/a:1008312726163. [DOI] [PubMed] [Google Scholar]
- [93].Jerusalem G, Beguin Y, Najjar F, et al. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin's lymphoma (NHL) Ann Oncol. 2001;12:825–30. doi: 10.1023/a:1011169332265. [DOI] [PubMed] [Google Scholar]
- [94].Bangerter M, Kotzerke J, Griesshammer M, et al. Positron emission tomography with 18-fluorodeoxyglucose in the staging and follow-up of lymphoma in the chest. Acta Oncol. 1999;38:799–804. doi: 10.1080/028418699432969. [DOI] [PubMed] [Google Scholar]
- [95].Sugawara Y, Zasadny KR, Kison PV, Baker LH, Wahl RL. Splenic fluorodeoxyglucose uptake increased by granulocyte colony-stimulating factor therapy: PET imaging results. J Nucl Med. 1999;40:1456–62. [PubMed] [Google Scholar]
- [96].Sugawara Y, Fisher SJ, Zasadny KR, et al. Preclinical and clinical studies of bone marrow uptake of fluorine-1-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol. 1998;16:173–80. doi: 10.1200/JCO.1998.16.1.173. [DOI] [PubMed] [Google Scholar]
- [97].Kazama T, Swanston N, Podoloff DA, Macapinlac HA. Effect of colony-stimulating factor and conventional- or high-dose chemotherapy on FDG uptake in bone marrow. Eur J Nucl Med Mol Imaging. 2005;32:1406–11. doi: 10.1007/s00259-005-1890-0. [DOI] [PubMed] [Google Scholar]
- [98].Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–61. doi: 10.1200/JCO.2005.01.891. [DOI] [PubMed] [Google Scholar]
- [99].Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- [100].Romer W, Hanauske AR, Ziegler S, et al. Positron emission tomography in non-Hodgkin's lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood. 1998;91:4464–71. [PubMed] [Google Scholar]
- [101].Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–9. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- [102].Schwartz DL, Rajendran J, Yueh B, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–7. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- [103].Kunkel M, Forster GJ, Reichert TE, et al. Detection of recurrent oral squamous cell carcinoma by [18F]-2-fluorodeoxyglucose-positron emission tomography: implications for prognosis and patient management. Cancer. 2003;98:2257–65. doi: 10.1002/cncr.11763. [DOI] [PubMed] [Google Scholar]
- [104].Laubenbacher C, Saumweber D, Wagner-Manslau C, et al. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI and endoscopy for staging head and neck squamous-cell carcinomas. J Nucl Med. 1995;36:1747–57. [PubMed] [Google Scholar]
- [105].Minn H, Lapela M, Klemi PJ, et al. Prediction of survival with fluorine-18-fluoro-deoxyglucose and PET in head and neck cancer. J Nucl Med. 1997;38:1907–11. [PubMed] [Google Scholar]
- [106].Brun E, Kjellen E, Tennvall J, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 2002;24:127–35. doi: 10.1002/hed.10037. [DOI] [PubMed] [Google Scholar]
- [107].Halfpenny W, Hain SF, Biassoni L, et al. FDG-PET. A possible prognostic factor in head and neck cancer. Br J Cancer. 2002;86:512–6. doi: 10.1038/sj.bjc.6600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Allal AS, Dulguerov P, Allaoua M, et al. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-D-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol. 2002;20:1398–404. doi: 10.1200/JCO.2002.20.5.1398. [DOI] [PubMed] [Google Scholar]
- [109].Oshida M, Uno K, Suzuki M, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer. 1998;82:2227–34. [PubMed] [Google Scholar]
- [110].Avril N, Menzel M, Dose J, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- [111].Patronas NJ, Di CG, Kufta C, et al. Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg. 1985;62:816–22. doi: 10.3171/jns.1985.62.6.0816. [DOI] [PubMed] [Google Scholar]
- [112].Choi JY, Jang HJ, Shim YM, et al. 18F-FDG PET in patients with esophageal squamous cell carcinoma undergoing curative surgery: prognostic implications. J Nucl Med. 2004;45:1843–50. [PubMed] [Google Scholar]
- [113].Fukunaga T, Okazumi S, Koide Y, Isono K, Imazeki K. Evaluation of esophageal cancers using fluorine-18-fluorodeoxyglucose PET. J Nucl Med. 1998;39:1002–7. [PubMed] [Google Scholar]
- [114].Nakata B, Nishimura S, Ishikawa T, et al. Prognostic predictive value of 18F-fluorodeoxyglucose positron emission tomography for patients with pancreatic cancer. Int J Oncol. 2001;19:53–8. doi: 10.3892/ijo.19.1.53. [DOI] [PubMed] [Google Scholar]
- [115].Sperti C, Pasquali C, Chierichetti F, et al. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953–9. doi: 10.1016/j.gassur.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [116].Zimny M, Fass J, Bares R, et al. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand J Gastroenterol. 2000;35:883–8. doi: 10.1080/003655200750023273. [DOI] [PubMed] [Google Scholar]
- [117].Nakata B, Chung YS, Nishimura S, et al. 18F-Fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer. 1997;79:695–9. [PubMed] [Google Scholar]
- [118].Shiomi S, Nishiguchi S, Ishizu H, et al. Usefulness of positron emission tomography with fluorine-18-fluorodeoxyglucose for predicting outcome in patients with hepatocellular carcinoma. Am J Gastroenterol. 2001;96:1877–80. doi: 10.1111/j.1572-0241.2001.03888.x. [DOI] [PubMed] [Google Scholar]
- [119].Antoch G, Kanja J, Bauer S, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–65. [PubMed] [Google Scholar]
- [120].Goerres GW, Stupp R, Barghouth G, et al. The value of PET, CT and in-line PET/CT in patients with gastrointestinal stromal tumours: long-term outcome of treatment with imatinib mesylate. Eur J Nucl Med Mol Imaging. 2005;32:153–62. doi: 10.1007/s00259-004-1633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- [122].Wieder HA, Brucher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–8. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]
- [123].Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–65. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- [124].Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–10. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- [125].Brun E, Kjellen E, Tennvall J, et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck. 2002;24:127–35. doi: 10.1002/hed.10037. [DOI] [PubMed] [Google Scholar]
- [126].Avril N, Sassen S, Schmalfeldt B, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol. 2005;23:7445–53. doi: 10.1200/JCO.2005.06.965. [DOI] [PubMed] [Google Scholar]