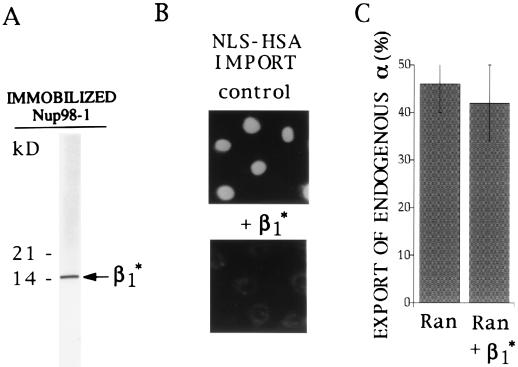
Figure 6.
Isolation of a 15-kDa tryptic fragment of karyopherin β1 that binds to the nucleoporin Nup98 and that inhibits import of NLS/HSA but not export of endogenous karyopherin α. (A) After limited trypsin digestion of recombinant karyopherin β1, a karyopherin β1 fragment of an estimated molecular mass of 15 kDa, termed β1*, was isolated by affinity chromatography on immobilized Nup98, eluted by 2 M NaCl and analyzed by SDS/PAGE and Coomassie blue staining. Numbers on the left indicate molecular mass markers. (B) Fluorescently labeled NLS/HSA was imported into nuclei of digitonin-permeabilized HeLa cells in the presence of 0.5 μM karyopherin α2, 0.3 μM karyopherin β1, 3 μM Ran, and 0.2 μM p10 (Upper) and in the additional presence of 40 μM β1*. (C) Export of endogenous karypherin α from digitonin-permeabilized HeLa cells was quantitatively analyzed as in Fig. 1B after a 30 min export reaction in the presence of 3 μM Ran alone or in the presence of 3 μM Ran and 40 μM β1*.