Summary
-
1
Rats have reached about 80% of the world's islands and are among the most successful invasive mammals. Rats are opportunistic predators that are notorious for their impact on a variety of animal and plant species. However, little documented evidence on the complexities of these interactions is available.
-
2
In our study, we assessed the impact of black rats Rattus rattus introduced on a small uninhabited island with a relatively simple ecosystem, Surprise Island, New Caledonia. We also compared the diet of R. rattus in the presence and absence of breeding seabirds, assessing the dietary compensation for this potentially important food source. From 2002 to 2005, we used live trapping studies combined with stable isotope analysis and conventional diet analyses (direct observations, gut and faecal contents) to characterize the diet of rats.
-
3
Our results suggest a heavy predatory impact on seabirds, which could constitute as much as 24% of the rat diet. Moreover, in the absence of birds, rats compensated marginally by preying more heavily on other components of their diet but mostly acquired a new resource. They shifted their diet by preying heavily upon another endangered species, the hatchlings of sea turtles Chelonia mydas, which could constitute the main resource in the diet of R. rattus in those periods. Abundance, body condition and distribution of the rats were consistent with heavy predation upon this additional resource.
-
4
Synthesis and applications. In island ecosystems invasive rats prey mainly upon seabird eggs and chicks, thereby threatening their populations. Although rats are certainly capable of surviving on terrestrial foods outside the seabird nesting season, their ability to prey upon ephemeral but abundant resources, such as hatchling sea turtles, may contribute to maintaining their populations. This may explain their success on Surprise Island, an ecosystem of extreme conditions, and suggests that biologists and managers working with threatened species should be aware of the possibility of temporary diet shifts by introduced rodents that may cause unexpected heavy predation on these species. This dietary shift from one endangered taxa to another has major implications for the conservation of seabirds and sea turtles world‐wide and more generally for the biodiversity of invaded insular communities.
Keywords: alien invasive species, Chelonia mydas, conventional diet, Rattus rattus, stable isotopes, trophic shift
Introduction
The invasion of oceanic islands by non‐native predators may lead to dramatic effects on island ecosystems (Atkinson 1985, 2001; Courchamp, Chapuis & Pascal 2003). Insular populations may be more vulnerable to predation not only because they are smaller and confined to fewer, specific habitats but also because they have evolved in the absence of predators and individuals may therefore lack anti‐predator adaptations exhibited by continental species (Dulloo, Kell & Jones 2002). Invasive predators, in contrast, are often ecological generalists that can successfully colonize a wide range of habitats on islands.
Introduced mammalian predators are implicated in about one‐half of island bird extinctions, for example in New Zealand and Hawaii. The Norway rat Rattus norvegicus Berkenhout, the black rat Rattus rattus Linnaeus and the Pacific rat Rattus exulans Peale have been introduced to more than 80% of the world's islands, including many uninhabited and inhospitable islands (e.g. arid islands with no water; Atkinson 1985). The three rat species have become widespread on islands with breeding bird colonies, probably because seabirds constitute a major source of animal food items: eggs, nestlings and even adult birds of many species are known to be predated by rats (Atkinson 1985; Robertson et al. 1998; Stapp 2002; Towns, Atkinson & Daugherty 2006). However, all three rats are opportunistic predators and on islands they also prey on some mammals (Donlan et al. 2003), reptiles (Cree, Daugherty & Hay 1995; Atkinson & Towns 2001; Towns, Atkinson & Daugherty 2006), arthropods (Robinet, Craig & Chardonnet 1998; Stapp 2002) and other invertebrates such as land snails (Towns, Atkinson & Daugherty 2006). Because of the naivety of many of these island organisms to predation by mammals and the consequential lack of behavioural, morphological and other life history anti‐predator responses, the impact of rats on island faunas and floras has been devastating, often leading to local or even global extinction (Atkinson 2001; Courchamp, Chapuis & Pascal 2003; Towns, Atkinson & Daugherty 2006).
Another factor that makes rats a particularly damaging alien species is their omnivorous diet; in addition to animal prey, they feed on roots, stems, leaves, flowers, fruits and seeds of a number of plant species (Campbell & Atkinson 2002; Towns, Atkinson & Daugherty 2006). This type of diet regarding managing alien species is problematic for two reasons. First, it increases the breadth of the diet, allowing the consumer to survive on several small populations of different species where a more specialized consumer would have died off. Secondly, it provides the consumer with the possibility of shifting from one resource type to another when one is absent, typically during the non‐breeding season of seabirds or when vegetation is poor (Atkinson 1985; Towns, Atkinson & Daugherty 2006). Although the impact of rats on insular species is widely acknowledged as important, little documented evidence is available to quantify the complexities of these interactions. Diet analyses of introduced rats are important in this regard.
Several techniques are available to study the diet of organisms, including direct observation of feeding behaviour, gut content and faecal analysis, and examination of chemical constituents such as stable isotopes. Each method provides a limited perspective but the results from different methods are often complementary. Conventional methods (direct observations and gut and faecal analysis) are useful for identifying specific prey taxa. However, there are several sources of bias when estimating diet component proportions with these methods, including the rapid digestion of soft‐bodied prey. Recently, numerous studies have used alternative approaches to traditional dietary analyses by measuring the proportional abundance of stable isotopes of various elements in different tissues from both consumer and potential prey species (Hobson & Clark 1993; Thompson et al. 1999). This approach is based on the fact that stable isotopic ratios of nitrogen (15N/14N, expressed as δ15N) and carbon (13C/12C, expressed as δ13C) in the consumer tissues reflect those in their prey in a predictable manner (DeNiro & Epstein 1978, 1981). Ratios of 13C (δ13C) in organisms generally reflect the isotopic composition of their diet, providing information on the original source of carbon in the food web. In contrast, 15N is consistently enriched in organisms at each trophic level, because organisms preferentially excrete the lighter nitrogen isotope (Minagawa & Wada 1984; Peterson & Fry 1987).
However, although the use of stable isotope analysis has a number of advantages over conventional methods in food web studies, the optimal approach is to combine it with conventional methods that provide a taxonomic resolution of resources (Vander Zanden, Cabana & Rasmussen 1997). Indeed, isotopic mixing models are very helpful in providing quantitative indices of food item contributions in a consumer's diet (Phillips & Gregg 2003) but they require prior classical diet analyses in order, among other things, to select the correct potential resource items.
The aim of this study was to assess the impact of black rats on an invaded island community. We selected a small, flat, uninhabited island with a relatively simple ecosystem in which the number of resource species available for rats was not excessive and in which other anthropogenic impact types were limited.
Our specific goals were to assess: (i) the diet of rats and their impact on different indigenous species, particularly seabirds; (ii) how rats adapt their diet outside the seabird breeding season; (iii) the repercussion of the rats’ diet compensation on their body condition, abundance and distribution in different areas. Finally, we discuss the importance of this diet compensation for the conservation of the implicated species.
Materials and methods
study site
The experiment was conducted on Surprise Island, Entrecasteaux Reefs, 230 km north of the main New Caledonia Island (Fig. 1). This remote island is a coral atoll of around 400 × 800 m (24 ha), 9 m above sea level. Temperature and rainfall define four seasons: a hot and humid season from December to March, when cyclones occur, and a cold and dry season from July to October, both separated by intermediate seasons (CTRDP 1987). Global positioning system (GPS) mapping revealed a central plain with different plant species (e.g. Graminae, Compositae and Portulaceae) surrounded by an arboreal strata with four dominant species, Argusia argentea Heine, Suriana maritima Arnott, Scaevola sericea Gaertn and Pisonia grandis Brown. We have listed around 40 different plant species, including coconut trees Cocos nucifera Linnaeus.
Figure 1.

Map of Surprise Island, d’Entrecasteaux Reefs, New Caledonia, showing the island core with two distinct vegetation zones (Plain and Shrubs and trees), surrounded by the seashore (Sands).
survey periods
The remoteness of the island and the difficult sea conditions during a large part of the year restricted the number of ships available for transportation and thus the number of possible visits. We therefore limited our visits to one per year from 2002 to 2005, each time for a similar period to allow interannual comparisons. We chose November, as most breeding bird species are present during this month (Robinet, Sirgouant & Bretagnolle 1997). In addition, because we wished to investigate how the rats might compensate for the absence of bird eggs and chicks during the hot humid season, we conducted a survey during the non‐breeding season in February 2005. A single field visit in February was sufficient to obtain predatory observations as well as data on isotopic values necessary for comparison with the November period. Because the cyclone season greatly limited the transport possibilities to and from this remote island, particularly at that time of the year, we had a very short stay of two nights, corresponding with a period which we believed would be the peak of hatchling sea turtle emergence (the sea turtle nesting period spans November–December).
fauna of surprise island
Surprise Island was inhabited from the 1890s for guano mining activities that continued until 1930. In 1965 an automatic meteorological station was established on Surprise Island (Pisier 1979). As a result of one or both anthropogenic activities, the island possesses an important suite of introduced species. The most important is the black rat but there is also a small population of mice Mus musculus Linnaeus on the central plain of the island. Two species of terrestrial reptile, probably introduced, are present: a skink Caledoniscincus haplorhinus Gunther, endemic to New Caledonia, and a gecko Lepidodactylus lugubris Dumeril and Bibron (Beugnet et al. 1993).
Surprise Island is a refuge for breeding marine vertebrates, including sea turtles and seabirds, both groups being very sensitive to human disturbance (Robinet, Sirgouant & Bretagnolle 1997). A total of 14 marine bird species was observed on Surprise Island, with 10 breeding on the island (Robinet, Sirgouant & Bretagnolle 1997; see below). Another bird species no longer present on Surprise Island since 2002, buff‐banded rail Gallirallus philippensis Linnaeus, is probably extinct because of rat predation (Robinet, Sirgouant & Bretagnolle 1997). Because all species do not breed simultaneously, there are breeding birds on the island during most of the year except January–April (Robinet, Sirgouant & Bretagnolle 1997).
Surprise Island provides nesting beaches for green turtles Chelonia mydas Linnaeus. During the hot and rainy season the turtles come to the beaches to lay eggs and the emergence of hatchling turtles occurs around 65 days later, with a peak in February. The vegetation hosts different arthropods (e.g. Coleoptera, Orthoptera, Acridae and Formicidae) and several species of marine crab live on the beach.
rat trapping
Line transects to trap rats were established in order to cover the whole island. Two zones could be distinguished: a ring next to the beach (hereafter called seashore) and a line across the island. We used Tomahawk live traps (type Institut National de Recherche Agronomique (INRA) rat traps 34 × 13 × 13 cm) baited with peanut butter. On the line transect across the island, trap stations were set every 25 m for two nights, resulting in 25–29 trap nights (number of traps × number of nights trapped), 25 in November 2002, 27 in November 2003, 26 in November 2004 and 2005 and 29 in February 2005). On the seashore transect, trap stations were spaced 50 m apart for four nights in November and two nights in February, resulting in 16–22 trap nights (20 trap nights in November 2002 and 2003, 16 in November 2004 and 22 in November 2005). In February 2005, trap stations on the seashore transect were spaced 10 m apart for 33 trap nights. In each instance, traps were opened in the late afternoon and checked and closed each morning.
We collected general information for each trap: we recorded whether a trap was sprung or not, the presence of bait and captures of rats. We calculated an index of rat abundance (IA) taking into account the number of corrected trap nights (Nelson & Clark 1973): IA = 100 × captures/(TU – S/2), where TU = P × N, P is the number of trapping intervals, N is the number of traps, S is the total number of traps sprung by any causes, TU is the number of trap nights and TU – S/2 is the number of corrected trap nights.
Captured rats were killed to collect tissue samples for stable isotope analysis and to examine gut and faecal contents. We examined the general appearance of all rats captured and the presence of ectoparasites (ticks). We noted whether the rats were normal or ‘mange’; the latter refers to a bad coat, particularly lack of hair. Different biometric parameters were recorded (e.g. the length of the right hind limb) as well as sex and sexual maturity.
gut and faecal contents
The stomach and faeces of rats were removed and washed and the contents were examined in the laboratory. The relative contributions of plant items and animal prey were estimated for each stomach and faecal sample under binocular lenses and a microscope. After 30 s of maceration in sodium hypochlorite, stomach and faecal samples were washed through a 0·25‐mm screen (Abbas 1988). For each of the stomach and faecal samples, five microscope slides were prepared and plant fragments were identified (Chapuis et al. 2001). We developed an extensive microphotographic collection of the epidermal tissues of the Surprise Island plant species (120 different items) as a reference for identifying plant fragments. Most epidermal fragments were identified to species level but no attempt was made to quantify the exact percentage composition of different species. For animal prey, different organs or identifiable fragments (e.g. legs, antenna and head capsule) were counted to estimate the minimum number of consumed prey items. A reference catalogue was also developed for animal prey, which could be used, in the case of arthropods, for identification to family level. Gut and faecal analyses were carried out on the November 2004 samples and compared with those of February 2005.
predation on seabirds and sea turtles
We also noted predation marks by rats on vegetation and animals (e.g. marks on egg shells and plants gnawed by rats) and direct observations of consumption and predation. In addition, we sought a quantitative estimate of the impact of rats on seabirds through egg predation. Because estimation of the number of eggs of tree‐nesting species, such as Sula sula Linnaeus, and burrow‐nesting species, such as Puffinus pacificus Gmelin, was too intrusive to be considered, we chose to focus on Sula dactylatra Lesson and Sula leucogaster Boddaert, two ground‐nesting species found only on the seashore and plain, respectively. We counted all nests with eggs or chicks (< 2 weeks old) for these two species in every visit to the island. In addition, in November 2003 we made two consecutive counts and noted the precise position of all eggs and chicks 1 week apart to estimate the predation rate by rats during the course of 1 week. When in the second count eggs and chicks were not present in the same place, we searched for them near the nest and noted possible evidence of rat predation on the egg shell or on the carcass of the chick. This allowed us to quantify the level of rat predation on the whole nesting population of both species on Surprise Island during 1 week. Because we studied this ecosystem over 4 years, we were familiar with the species and their interactions; there were no other potential predators of the eggs and chicks on Surprise Island. In December 2002 we counted (over 13 days) the number of nest marks made by individual adult female Chelonia mydas Linnaeus coming onto the beach to lay eggs, to estimate the abundance of turtle nests. Finally, in February 2005 we surveyed sea turtle emergences over two consecutive nights, continuously patrolling the seashore to observe the fate of hatchling sea turtles heading to the sea.
stable isotope analysis
Samples from livers of captured rats and samples from potential rat food items were collected for stable isotope analysis. We selected the liver tissue from rats because the turnover rates of stable isotopes are high and reflect recent diet (about 1 week; Tieszen et al. 1983; Hobson & Clark 1992a,b). We used samples from two consecutive periods, the November 2004 and February 2005 surveys, to compare the diet of rats during and outside the seabird breeding season. Samples were kept in alcohol until they were freeze‐dried and ground to a fine powder. Dried samples were weighed into tin capsules and stored in a desiccator until measurement. Isotopic analyses were performed by a spectrometer IsoPrime (MicroMass, Institut de Biotechnologie des Plantes, Université Paris Sud, Paris, France) coupled with analyser EuroEA 3024 (EuroVector, Institut de Biotechnologie des Plantes, Université Paris Sud, Paris, France). Stable carbon (C) and nitrogen (N) isotope ratios are expressed as δ13C or δ15N = [(R sample/R standard) – 1] × 1000, where R is 13C/12C or 15N/14N for δ13C or δ15N, respectively. The standard for C is IAEA‐NBS 21 (graphite, 28·13‰) and for N IAEA‐N1 (+0·4‰) and IAEA‐N2 (+20·3‰). Ten replicate assays of internal laboratory standards indicated maximum measurement errors (SD) of ±0·15‰ and ±0·2‰ for stable C and N isotope measurements, respectively.
Phillips & Gregg (2003) developed the Isosource model, which calculates the range of all possible source contributions for systems where the number of potential sources is greater than n+ 1, n being the number of isotopes. Isotopic models typically use the mean δ13C and δ15N values for each type of diet, corrected for the discrimination factor of the consumer (the increase in consumer isotopic ratio compared with its diet, noted as ΔN and ΔC). Discrimination factors depend on several sources of variation (e.g. taxon, environment and tissue). Previous laboratory work had shown significant relationships between δ13C and δ15N of diets and the corresponding ΔN and ΔC of the different tissues of rats fed on these diets (see Appendix S1 in the supplementary material). We thus calculated, for the rat liver, the diet‐dependent discrimination factors corresponding to each potential rat diet item (Table 1). We then ran the Isosource model with a source increment of 1% and a mass balance tolerance of ±0·1‰.
Table 1.
Discrimination factors (ΔC and ΔN) for rat livers calculated with the diet‐dependent discrimination factor method for each rat food item (See Appendix S1). These discrimination factors were used for analysis with the Isosource isotopic model (Phillips & Gregg 2003)
| Rat food items | November 2004 | February 2005 | ||
|---|---|---|---|---|
| ΔC | ΔN | ΔC | ΔN | |
| Skinks | –1·77 | –0·03 | –0·36 | 0 |
| Insects (I) | –2·89 | 0·80 | –3·43 | 1·56 |
| Insects (II) | 1·07 | 0·80 | 1·22 | 0·87 |
| Plants C4/CAM | –2·78 | 2·09 | –2·81 | 2·15 |
| Plants C3 | 1·21 | 2·73 | 0·75 | 2·80 |
| Sea turtles | –1·65 | 2·26 | ||
| Seabirds | –0·84 | 1·49 | ||
statistical analysis and hypotheses tested
We tested differences in body condition, rat capture rates and rat isotopic values between survey periods, specifically between the February 2005 survey (in the absence of seabirds) and the November surveys (in their presence). Dependent variables were tested for normality and we used either generalized linear models with binomial distribution and logit link function (GLMB) when the response variable was an occurrence or a rate, or general linear models (GLMN) when the response variable was distributed normally. The main independent variable was the survey period, a categorical variable with five levels (November 2002, November 2003, November 2004, February 2005 and November 2005). Differences between periods were explored using planned comparisons, as we wanted to know whether mean parameters of the February survey were different from mean parameters of all November surveys. Computations were performed with statistica 6.0 (StatSoft Inc. 2001) except generalized linear models, which were performed with the SAS package (genmod version 9.1.3; SAS Institute Inc. 2004).
Even in omnivorous consumers, we can suppose that body condition changes if the availability of the main prey is reduced and not compensated for by alternative prey; this is particularly likely in small, waterless insular ecosystems. We tested whether body condition differed between rats in the February and November surveys. Body condition was measured with three different indices: (i) a body condition index resulting from the residuals of a linear regression of mass against the length of the right hind limb (Green 2001); (ii) the occurrence of mange; (iii) the occurrence of ticks. The occurrence of mange and ticks in the trapped rats was analysed with GLMB while the body condition index was analysed with GLMN. In the body condition index GLMN we added two more independent variables, the sex of the rat and the distance to the sea (assuming that rats closer to the sea could encounter a higher availability of hatchling turtles and rats further from the sea could encounter a greater availability of seabird eggs).
In insular ecosystems, changes in the availability of different prey could lead to changes in predator abundance or home range. We tested for differences in the capture rates of rats (×10, to convert into integer) between the February and November survey periods and between zones. We performed two GLMB, one for each zone (seashore and across the island).
A shift in rat diet could be reflected by a shift in the isotopic values of rat tissues. We tested whether isotopic ratios of carbon and nitrogen in the liver of dead rats differed between surveys using GLMN.
Results
body condition, abundance and distribution of rats
The occurrence of ticks showed no significant differences between surveys (χ2 = 0·32, P= 0·573, n= 277); the occurrence of mange, however, showed differences between surveys (χ2 = 6·41, P= 0·011, n= 277). No rats were seen with mange in February. Differences between surveys can be seen in Fig. 2a.
Figure 2.
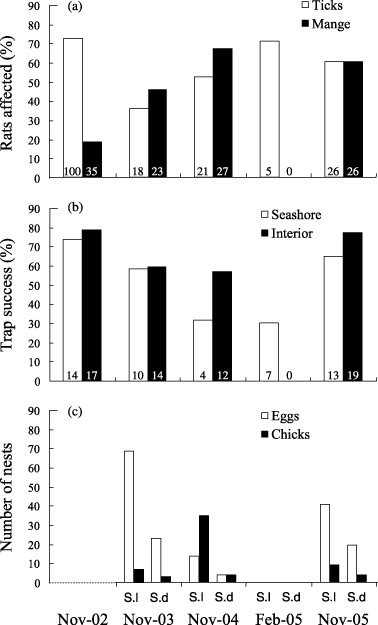
Rat body condition, rat capture rates and prey availability in the different survey periods: (a) percentage of trapped rats with mange and ticks; (b) percentage of corrected trap nights with at least one rat caught in the two zones, seashore and across the island (interior); (c) number of nests of Sula leucogaster (S.l) and Sula dactylatra (S.d) with at least one egg or one chick (this survey was not done in November 2002). The number of rats is marked in italics inside each bar.
The body condition index was only influenced by sex (F 1,188 = 14·72, P= 0·0002) and not by survey periods or distance to the sea (F 4,188 = 2·01, P= 0·094; F 1,188 = 0·24, P= 0·625, respectively).
Capture rates showed significant differences between survey periods in the two zones examined (χ2 = 118·52, P < 0·0001, n= 91·5 on the seashore and χ2 = 429·85, P < 0·0001, n= 110·5 across the island). Planned comparisons showed that the February survey was significantly different to the November surveys in both island zones (Fig. 2b). No rats were captured across the island in the February survey. Differences in captures rates were assumed to reflect quantitative differences in the abundance of rats between zones.
food habits of rats
The analysis of stomach contents revealed that rats consumed a wide variety of food items (Table 2). Plants were found in the guts and faeces of 100% (n = 16) of the rats collected in November and 67% (n = 6) of the rats collected in February. We distinguished two types of plants based on their isotopic values: C3 plants, which use the enzyme Rubisco to fix CO2, and C4 plants, which fix CO2 with phosphoenolpyruvate carboxylase. In November, we found eight species of C3 plants with one of them, Pisonia grandis, occurring in 94% of the rats, and five species of C4 plants with one species, Boerhavia repens Linnaeus, occurring in 88% of the rats. In February, we found only three C3 plant species and three C4 plant species, but with the same major species as in November, P. grandis and B. repens, respectively. However, sample sizes in the February survey were reduced (six rats examined compared with 16 from November).
Table 2.
Percentage occurrence of food items in the guts (G) and faeces (F) of Rattus rattus from Surprise Island in November 2004 and February 2005. Direct observations of predation (rat teeth marks and consumption by rats, DO) are noted by the symbol +. n represents sample size for gut and faecal contents corresponding to the number of rats examined
| Rat food items | November 2004 (n = 16) | February 2005 (n = 6) | ||||
|---|---|---|---|---|---|---|
| G | F | DO | G | F | DO | |
| Seabird feathers | 56 | 38 | 0 | 0 | ||
| Seabird eggs | 0 | 0 | + | 0 | 0 | |
| Sea turtles | 0 | 0 | 0 | 0 | + | |
| Skinks | 13 | 0 | 0 | 0 | ||
| Insects (I) | 38 | 75 | 83 | 67 | ||
| Insects (II) | 50 | 100 | 67 | 50 | ||
| Plants C3 | 100 | 100 | 50 | 67 | ||
| Achyrantes aspera | 13 | 44 | 0 | 0 | ||
| Argusia argentea | 13 | 25 | + | 33 | 33 | + |
| Tridax procubens | 13 | 31 | + | 0 | 17 | |
| Abutilon sp. | 6 | 6 | 0 | 0 | ||
| Hibiscus tiliacens | 6 | 0 | 0 | 0 | ||
| Pisonia grandis | 75 | 94 | + | 50 | 50 | + |
| Cocos nucifera | 0 | 0 | + | 0 | 0 | + |
| Colubrina asiatica | 12 | 18 | 0 | 0 | ||
| Microsorum scolopendrium | 6 | 0 | 0 | 0 | ||
| Plants C4/CAM | 75 | 100 | 33 | 50 | ||
| Portulaca sp. | 6 | 50 | + | 17 | 17 | |
| Boerhavia repens | 44 | 88 | + | 17 | 33 | + |
| Tribulus cistoides | 12 | 18 | + | 0 | 0 | |
| Lepturus repens | 25 | 25 | 0 | 33 | ||
| Stenatophrum micrathum | 6 | 18 | 0 | 0 | ||
According to the isotopic values, we distinguished two categories of insects (I, Orthoptera, and II, Coleoptera and Lepidoptera) that were observed in large proportions in the gut and faeces of trapped rats in both November and February (Table 2). Skinks were collected from the gut contents of 13% of the rats captured in November but were not seen in the limited sample from February. Seabird feathers were found in the guts of 56% of rats captured in November and in 0% of the February samples.
Direct observations in the field (both of rat teeth marks and consumption by rats) allowed us to confirm the analysis of gut and faecal contents, particularly for plants. We observed an important consumption of roots (Boerhavia repens and Portulaca sp.) and stems (Argusia argentea, Pisonia grandis) that were difficult to identify as fragments in faeces and gut contents. All coconuts were gnawed and this was probably an important source of water for the rats.
predation on seabirds and sea turtles
The most important difference in availability of food items between November and February was the presence on the island of eggs and chicks of seabirds in all three November periods but not in February (Fig. 2c). Each November period, we observed marks of rat predation on the eggs of Sula leucogaster, Sula dactylatra and Puffinus pacificus (Fig. 3a). In addition, we observed seabird feathers in the gut and faeces of rats in the November surveys. In November 2003, 26·1% (n = 69) of S. leucogaster eggs and 0% (n = 10) of S. dactylatra eggs were predated in 1 single week.
Figure 3.

Observations of rat predation on different items on Surprise Island: (a) teeth marks on a predated egg of Sula leucogaster observed in November 2004; (b) a killed hatchling of Chelonia mydas observed in February 2005.
No eggs or chicks were found in February (Fig. 2c), which was not surprising given that the nesting season had ended a few weeks earlier with the fledging of aged chicks (Robinet, Sirgouant & Bretagnolle 1997). However, a new food item was present in this period: hatchlings of the green turtle. Our survey of turtle nest marks in December 2002 revealed a total of 806 nests with a maximum of 82 nests in one night. Direct observations during two survey nights in February 2005 were sufficient to report evidence of direct predation by rats on hatchling turtles. In particular, we observed a rat stalking, catching and eating a hatchling. The rat showed a particularly agitated behaviour at the sight of hatchlings. It made a quick series of vertical hops before attacking the hatchlings, suggesting the prey item was known. The turtle was caught and eaten alive. Several half‐eaten turtles were found the same night (Fig. 3b). However, we did not find fragments of sea turtles in the guts or faeces of rats, probably because the number of rats was not sufficient in the February survey (n = 6).
isotopic analysis
Conventional diet analyses and observations allowed the selection of different prey as inputs for isotopic models (Table 2). We used the following food items to run isotopic models for the November 2004 survey: the nine most commonly eaten species of C3 plants, the five major species of C4 plants, commonly found insects (of isotopic categories I and II), skinks and seabirds. For the February 2005 survey, we used four species of C3 plants, three species of C4 plants, insects (isotopic categories I and II), skinks and sea turtles. We chose to use only the plant species observed in February 2005, even if the number of rats captured was small, because the standard deviation for plants was very low (Fig. 4). Although we did not find it in the gut or faecal contents, we included skink in the isotopic model for the February diet. We assumed that it is a sporadic but large item that could be difficult to find in a small sample of rat stomachs and saw no reason to assume that rats would ignore such a major November food item when it was also very abundant in February.
Figure 4.
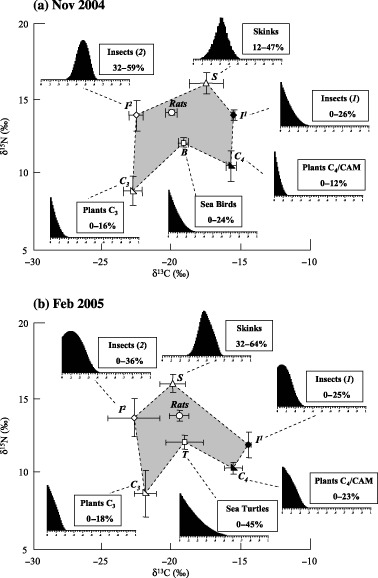
δ13C and δ15N signatures (± SE) of six major rat prey types on Surprise Island (after correction with the discrimination factors). The polygon (light grey) is circumscribed by the isotopic signatures of the prey types, the signature of the rat being in its centre. Histograms show the distribution of feasible contributions from each prey to the rat diet resulting from the application of the Isosource isotopic model. Values shown in the boxes are 1–99 percentile ranges for these distributions. Comparison between November (a) and February (b) shows that seabirds and sea turtles have comparable isotopic values, generating only a minor shift in the rat values between the two periods.
Rat isotopic ratios showed differences between survey periods (F 4,59 = 6·46, P= 0·0002 for carbon and F 4,59 = 29·27, P < 0·0001 for nitrogen). Planned comparisons showed significant differences between mean isotopic values of rats captured in the February 2005 survey and the mean of all isotopic values of rats captured in the November surveys for both isotopes.
Results from stable isotopic models depicted proportions of food in the diet of rats that were concordant with food habit information derived from conventional diet analysis. In both periods, all six preys formed a polygon in the centre of which was the rat (Fig. 4). The results for November 2004 and February 2005 showed comparable proportions for C3 plants (0–16% in November and 0–17% in February) and insects I (0–26% vs. 0–25%), an increase of C4/CAM plants (0–12% vs. 0–23%) and skinks (12–47% vs. 32–64%) and a decrease of insects II (32–59% vs. 0–36%). But the most striking feature of the diet change was the replacement of seabirds (0–24% in November) by sea turtles (0–45% in February), which became the second most important food item, immediately after skinks (considering the maximum possible contribution). Most notably, seabirds and sea turtles had comparable values of δ13C and δ15N.
Discussion
Using a combination of classic and isotopic analyses, we found that rats on invaded Surprise Island had a wide diet. At least three species of seabird are a major prey of rats and are probably threatened by the level of rat predation. In the absence of breeding birds, rats compensate for the loss of this food source by preying more heavily on skinks and, particularly, by preying on a new prey item, sea turtle hatchlings. This shift between two temporary food resources could help to maintain a population of rats in a very inhospitable habitat, thereby maintaining the threat to each prey species. Congruent with an efficient use of resources, rats did not exhibit a decrease in body condition in the absence of birds.
conventional and isotopic analyses
Animal diets determined by foraging observations, gut contents and faecal analysis are often difficult to interpret (Witmer et al. 2006). The data obtained by these methods are restrictive because they only provide information about what an animal has eaten during a recent, brief window of time (i.e. what is in the gut at the time the animal was captured or what the animal was seen eating). There are also observational biases associated with such data because it is often easier to see animals feeding on one type of resource than on another. Stable isotopes offer an alternative method for reconstructing diets and evaluating the relative importance of dietary components to consumers. Isotopic models are powerful tools but the validity of the results depends on how well their assumptions are met. For example, the mixing model method implicitly assumes that a consumer is in isotopic equilibrium with its diet (Phillips 2001). This assumption is problematic, particularly after a diet switch, because the isotopic values of the consumer change gradually. To overcome these problems we: (i) chose rat tissue with a high turnover rate, the liver, that may reflect diet assimilated over the previous week (Hobson & Clark 1992a, 1992b); (ii) calculated discrimination factors with regression taking into account the isotopic value of each source (see Appendix S1 in the supplementary material); (ii) used conventional diet study methods to define the source inputs for the isotopic models.
Our results revealed that rats on Surprise Island predated seabirds. This has previously been demonstrated by Stapp (2002), who studied the diet of black rats on the Shiant Islands using this technique, and previous guts and faecal analyses have revealed that the presence of feathers is not uncommon in the rat (Key et al. 1998; Stapp 2002). But neither technique can distinguish between predation and scavenging, which can only be done by observation or repeated nest counting experiments such as ours. We found no evidence of sea turtle hatchlings in the guts of rats, which was probably because of the low number of rats trapped in the February survey (n = 6). However, active predation was directly observed. This is, to our knowledge, the first report of direct predation of sea turtle hatchlings by rats (but see Witmer, Boyd & Hillis‐Starr 2007).
diet plasticity of invaders to exploit an ephemeral resource
It has been speculated that alien rodents shift their diet proportions in order to compensate when one food item is temporarily absent (Imber, Harrison & Harrison 2000; Towns, Atkinson & Daugherty 2006; Witmer, Boyd & Hillis‐Starr 2007). We observed a diet shift over 3 months (between November and February). The rat population was probably the same over this period, as rats captured in February were clearly adults. Our result not only confirms the hypothesized temporal plasticity of rat diets but also shows that it extends to the inclusion of new resources that are only available during the shift period. The comparison of the number of rats captured per trap night confirmed the movement of rats from the centre of the island (where eggs were predated) in November, to the seashore (where hatchlings were predated) in February. Even if the spacing between traps differed on the seashore between the February and November surveys, this may not have directly affected the conclusions; what is important is the absence of rats in the interior. Moreover, the numbers of rats captured in February may lead to an underestimation of the population because traps covered a smaller surface area (traps were spaced at 10 m in February compared with 50 m in November). The capacity of generalist predators to switch their diet opportunistically to temporarily abundant food sources has long been hypothesized (Wilson & Turelli 1986; Hanski et al. 2001) and recently demonstrated (Popa‐Lisseanu et al. 2007) as an important ecological advantage for exploiting new niches. This plasticity could explain the success of invasive rats throughout the world. Stapp (2002) suggested, and we have now demonstrated, that marine resources subsidize insular rat populations (permitting higher densities than would be possible based on terrestrial resources alone).
rat predation on seabirds and sea turtles
Rats are known to kill and eat adults of some small species of seabirds, but in most cases predation by rats tends to be on eggs and young when these are available (Atkinson 1985). On Surprise Island, direct observations confirmed an impact of rats on the eggs of seabirds (Fig. 3a), at least in three of the 10 breeding species. In just 1 week in 2004, more than 25% of all eggs of the Sula leucogaster population of Surprise Island were predated by rats. We did not find the same result for Sula dactylatra, probably because adults remained on the nest and were aggressive while defending their eggs.
Because of the logistic restrictions discussed above, we could not directly quantify predation on sea turtles. Yet stable isotope analysis complemented direct observation by revealing the importance of sea turtles as a seasonal food source: sea turtles could constitute as much as 45% of the rat diet in the hatching season, while seabirds constituted as much as 24% during their breeding season. The hatching success of Chelonia mydas is in general 85% and they lay an average of 110 eggs (Miller 1997). In December 2002 on Surprise Island, we observed a total of 806 nests with a maximum of 82 nests in one night. If around 90 hatchlings emerged per nest, emergences of sea turtles represent a potential and abundant food source. According to this, sea turtles seem to be a more abundant food than seabird eggs, as a maximum of 151 nests of Sula leucogaster, normally with two eggs per nest, were available during their breeding season. Yet the proportionate impact on the bird populations, which are less numerous and lay fewer eggs, would presumably be greater.
The results presented here confirm that active predation by rats plays an important role but the historical impact of rat presence on Surprise Island's seabirds and sea turtles remains unquantified. The presence of rats on Surprise Island probably limits opportunities for the establishment and growth of breeding populations of small‐bodied ground‐nesting seabirds such as Anous tenuirostris Temminck, Anous stolidus Linnaeus, Sterna sumatra Raffles, Sterna bergii Lavery, Sterna fuscata Linnaeus and Sterna anaethetus Linnaeus, which are particularly susceptible to rat predation (Towns, Atkinson & Daugherty 2006). The d’Entrecasteaux Reefs include two atolls, one with three islets (Surprise, Fabre and Le Leizour Islands) and the other with one (Huon Island). In November 2005 we surveyed these islands for comparison with the Surprise Island ecosystem. We observed ground‐nesting seabird species that were not breeding on Surprise Island (Anous tenuirostris Temminck, Anous stolidus Linnaeus, Sterna sumatra Raffles, Sterna bergii Lavery, Sterna fuscata Linnaeus and Sterna anaethetus Linnaeus). We found no introduced rodents on these islands (15 trap nights for rats and 15 trap nights for mice on each island). We concluded that the presence of introduced rats on Surprise Island for around 100 years has dramatically impacted these colonies; some species do not reproduce (Sterna sumatra, Sterna bergii, Sterna fuscata and Sterna anaethetus), one species has declined dramatically (Phaeton rubricauda Bodd) and another species has been extirpated (Gallirallus philippensis).
synthesis and applications
Our study shows that (i) introduced rats are important predators of insular species, in particular eggs and chicks of seabirds; (ii) outside the seabird breeding season, they shift their diet and show no sign of being affected by the loss of this major resource; (iii) in addition to adjusting the proportion of remaining resources, rats can compensate by preying upon a new, ephemeral but abundant, resource, sea turtle hatchlings, thus switching from predation of one endangered taxa to another; and (iv) although isotopic analyses played a crucial role in the provision of these results, it could not have produced them alone, calling for the systematic use of combined diet analysis methods for the study of alien predator impacts on invaded ecosystems. The sheer number of eggs and chicks predated in 1 single week of observation demonstrates the magnitude of the impact that invasive black rats can have on insular avifaunas. Although it might have been the peak of predation on this species, or an exceptional year, this figure undoubtedly calls for action to protect local species.
The demonstration of a shift between two temporally heterogeneous groups of prey is an important concern for biological conservation because prey populations are often endangered (globally or locally) and potentially highly impacted by rat predation. The confirmed predation of sea turtle hatchlings by rats raises major concerns for managers and conservationists of marine turtles world‐wide, given the ubiquity of introduced rats on islands. Moreover, biologists and managers working with threatened species should be aware of the possibility of temporary shifts in introduced rodent diets that may cause unexpected heavy predation on these species.
Supporting information
Appendix S1. Relationship between isotopic ratio of prey and discrimination factors of carbon and nitrogen for the rat liver.
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgements
We are grateful to the many colleagues who helped us in the field collecting data on Surprise Island, especially X. Cerda and J. L. Chapuis. We wish to thank Richard Hall and Donna Harris for comments on the manuscript and improvement of the English. This research is part of a research and conservation program financed by grants from IFB, INSU (ACI ECCO‐PNBC), ANR and the Government of New Caledonia. All authors have applied appropriate ethics and other approval for the research; S. Caut was authorized for animal experimentation (R‐45GRETA‐F1‐04) by the French Minister of Agriculture. E. Angulo was supported by a postdoctoral fellowship from the Spanish Ministry of Education and Science (SEEU‐FSE).
Re‐use of this article is permitted in accordance with the Creative Commons Deed, Attribution 2·5, which does not permit commercial exploitation.
References
- Abbas, A. (1988) Régime alimentaire d’un phytophage introduit, le ragondin (Myocastor coypus Molina 1782), dans differents types de marais amenages . PhD Thesis. University of Rennes I, Rennes, France. [Google Scholar]
- Atkinson, I.A.E. (1985) The spread of commensal species of Rattus to oceanic islands and their effect on island avifaunas. Conservation of Island Birds, 3, 35–81. [Google Scholar]
- Atkinson, I.A.E. (2001) Introduced mammals and models for restoration. Biological Conservation, 99, 81–96. [Google Scholar]
- Atkinson, I.A.E. & Towns, D.R. (2001) Advances in New Zealand mammalogy 1990–2000: Pacific rat. Journal of the Royal Society of New Zealand, 31, 99–109. [Google Scholar]
- Beugnet, F. , Costa, R. , Ferre, O. & Marchal, V. (1993) Ecologic and pathological studies of seabirds from French South‐Pacific Islet: Isle Surprise (18°29′ S, 162°05′ E) as example. Revue de Medecine Veterinaire, 144, 607–613. [Google Scholar]
- Campbell, D.J. & Atkinson, I.A.E. (2002) Depression of tree recruitment by the Pacific rat (Rattus exulans Peale) on New Zealand's northern offshore islands. Biological Conservation, 107, 19–35. [Google Scholar]
- Chapuis, J.L. , Bousses, P. , Pisanu, B. & Reale, D. (2001) Comparative rumen and fecal diet microhistological determinations of European mouflon. Journal of Range Management, 54, 239–242. [Google Scholar]
- Courchamp, F. , Chapuis, J.L. & Pascal, M. (2003) Mammal invaders on islands: impact, control and control impact. Biological Reviews, 78, 347–383. [DOI] [PubMed] [Google Scholar]
- Cree, A. , Daugherty, C.H. & Hay, J.M. (1995) Reproduction of a rare New‐Zealand reptile, the Tuatara sphenodon‐punctatus, on rat‐free and rat‐inhabited islands. Conservation Biology, 9, 373–383. [Google Scholar]
- CTRDP (1987) Ecologie en Nouvelle Calédonie. Centre Territorial de Recherche et de Documentation, Nouméa, New Caledonia. [Google Scholar]
- DeNiro, M.J. & Epstein, S. (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Cosmochimica Acta, 42, 495–506. [Google Scholar]
- DeNiro, M.J. & Epstein, S. (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta, 45, 341–351. [Google Scholar]
- Donlan, C.J. , Howald, G.R. , Tershey, B.R. & Croll, D.A. (2003) Evaluating alternative rodenticides for island conservation: roof rat eradication from the San Jorge Islands, Mexico. Biological Conservation, 114, 29–34. [Google Scholar]
- Dulloo, M.E. , Kell, S.R. & Jones, C.G. (2002) Impact and control of invasive alien species on small islands. International Forestry Review, 4, 277–285. [Google Scholar]
- Green, A.J. (2001) Mass/length residuals: measures of body condition or generators of spurious results? Ecology, 82, 1473–1483. [Google Scholar]
- Hanski, I. , Henttonen, H. , Korpimaki, E. , Oksanen, L. & Turchin, P. (2001) Small‐rodent dynamics and predation. Ecology, 82, 1505–1520. [Google Scholar]
- Hobson, K.A. & Clark, R.G. (1992a) Assessing avian diets using stable isotopes. II. Factors influencing diet–tissue fractionation. Condor, 94, 189–197. [Google Scholar]
- Hobson, K.A. & Clark, R.G. (1992b) Assessing avian diets using stable isotopes. I. Turnover of C‐13 in tissues. Condor, 94, 181–188. [Google Scholar]
- Hobson, K.A. & Clark, R.G. (1993) Turnover of C‐13 in cellular and plasma fractions of blood: implications for nondestructive sampling in avian dietary studies. Auk, 110, 638–641. [Google Scholar]
- Imber, M. , Harrison, M. & Harrison, J. (2000) Interactions between petrels, rats and rabbits on Whale Island, and effects of rat and rabbit eradication. New Zealand Journal of Ecology, 24, 153–160. [Google Scholar]
- Key, G. , Fielding, A.H. , Goulding, M.J. , Holm, R.S. & Stevens‐Woods, B. (1998) Ship rats Rattus rattus on the Shiant islands, Hebrides, Scotland. Journal of Zoology, 245, 228–233. [Google Scholar]
- Miller, J.D. (1997) Reproduction in sea turtles The Biology of Sea Turtles (eds Lutz P.L. & Musick J.A.), pp. 51–82. CRC Press, Boca Raton, FL. [Google Scholar]
- Minagawa, M. & Wada, E. (1984) Stepwise enrichment of N‐15 along food chains: further evidence and the relation between delta‐N‐15 and animal age. Geochimica et Cosmochimica Acta, 48, 1135–1140. [Google Scholar]
- Nelson, L.J. & Clark, F.W. (1973) Correction for sprung traps in catch/effort calculation of trapping results. Journal of Mammals, 54, 295–298. [Google Scholar]
- Peterson, B.J. & Fry, B. (1987) Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics, 18, 293–320. [Google Scholar]
- Phillips, D.L. (2001) Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia, 127, 166–170. [DOI] [PubMed] [Google Scholar]
- Phillips, D.L. & Gregg, J.W. (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia, 136, 261–269. [DOI] [PubMed] [Google Scholar]
- Pisier, G. (1979) Notes d’histoire Calédonienne. Les ‘petites dépendences’ de la Nouvelle Calédonie. Bulletin de la Société d’Etudes et d’histoire de Nouvelle Calédonie, 41, 9–32. [Google Scholar]
- Popa‐Lisseanu, A.G. , Delgado‐Huertas, A. , Forero, M.G. , Rodríguez, A. , Arlettaz, R. & Ibáñez, C. (2007) Bats’ conquest of a formidable foraging niche: the myriads of nocturnally migrating songbirds. PLoS ONE, 2, e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, A. , Jarvis, A.M. , Brown, C.J. & Simmons, R.E. (1998) Avian diversity and endemism in Namibia: patterns from the southern African bird atlas project. Biodiversity and Conservation, 7, 495–511. [Google Scholar]
- Robinet, O. , Craig, J.L. & Chardonnet, L. (1998) Impact of rat species in Ouvea and Lifou (Loyalty Islands) and their consequences for conserving the endangered Ouvea parakeet. Biological Conservation, 86, 223–232. [Google Scholar]
- Robinet, O. , Sirgouant, S. & Bretagnolle, V. (1997) Marine birds of d’Entrecasteaux Reefs (New Caledonia, southwestern Pacific): diversity, abundance, trends and threats. Colonial Waterbirds, 20, 282–290. [Google Scholar]
- SAS Institute Inc . (2004) SAS, 9·1·3, Help and Documentation. SAS Institute Inc, Cary, NC. [Google Scholar]
- Stapp, P. (2002) Stable isotopes reveal evidence of predation by ship rats on seabirds on the Shiant Islands, Scotland. Journal of Applied Ecology, 39, 831–840. [Google Scholar]
- StatSoft Inc . (2001) Data Analysis Software System, Version 6. StatSoft Inc; http://www.statsoft.com . [Google Scholar]
- Thompson, D.R. , Lilliendahl, K. , Solmundsson, J. , Furness, R.W. , Waldron, S. & Phillips, R.A. (1999) Trophic relationships among six species of Icelandic seabirds as determined through stable isotope analysis. Condor, 101, 898–903. [Google Scholar]
- Tieszen, L.L. , Boutton, T.W. , Tesdahl, K.G. & Slade, N.A. (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia, 57, 32–37. [DOI] [PubMed] [Google Scholar]
- Towns, D.R. , Atkinson, I.A.E. & Daugherty, C.H. (2006) Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions, 8, 863–891. [Google Scholar]
- Vander Zanden, M.J. , Cabana, G. & Rasmussen, J.B. (1997) Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (delta N‐15) and literature dietary data. Canadian Journal of Fisheries and Aquatic Sciences, 54, 1142–1158. [Google Scholar]
- Wilson, D.S. & Turelli, M. (1986) Stable underdominance and the evolutionary invasion of empty niches. American Naturalist, 127, 835–850. [Google Scholar]
- Witmer, G.W. , Boyd, F. & Hillis‐Starr, Z. (2007) The successful eradication of introduced roof rats (Rattus rattus) from Buck Island using diphacinone, followed by an irruption of house mice (Mus musculus ). Wildlife Research, 34, 108–115. [Google Scholar]
- Witmer, G.W. , Burke, P. , Jojola, S. & Dunlevy, P. (2006) The biology of introduced Norway rats on Kiska Island, Alaska, and an evaluation of an eradication approach. Northwest Science, 80, 191–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Relationship between isotopic ratio of prey and discrimination factors of carbon and nitrogen for the rat liver.
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


