Abstract
The role of antigen-presenting cells (APC) in the induction of antigen-specific unresponsiveness was examined, using two functionally distinct murine macrophage hybridomas, #59 and #63 cells. Derivatized with the hapten (dinitrofluorobenzene; DNFB), #59 cells induced contact hypersensitivity (CH) in mice. Hapten-derivatized #63 cells failed to induce CH. Instead, they prevented recipients from acquiring CH when exposed subsequently to a sensitizing dose of the hapten. Similarly, hapten-derivatized #59 cells, pretreated in vitro with transforming growth factor-β2 (TGF-β2) lost their capacity to evoke CH, and induced tolerance. Hapten-derivatized #63 cells and TGF-β2-treated #59 cells eliminated CH in mice sensitized to hapten. Reverse transcription–polymerase chain reaction analysis of mRNAs for various accessory molecules important in T-cell activation revealed that #63 and TGF-β2-treated #59 cells differed only in their expression of tumour necrosis factor-α (TNF-α) mRNA. The latter expressed higher levels of TNF-α mRNA than did untreated #59 cells. As a consequence, #63 and TGF-β2-treated #59 cells, both of which induce tolerance, secrete TNF-α protein unlike untreated #59 cells, which do not induce tolerance to hapten. Since neutralizing anti-TNF-α antibodies abrogated the tolerogenic potential of #63 cells in vivo, we conclude that TGF-β2 equips hapten-bearing APC with the capacity to evoke systemic immune deviation in which CH is selectively silenced. We speculate that one effect of TGF-β2 is to cause APC to up-regulate TNF-α production. In turn, this cytokine biases the functional property of responding hapten-specific T cells in a direction that not only interferes with acquisition, but suppresses induction of CH.
INTRODUCTION
Immunological tolerance refers to states of antigen-specific unresponsiveness achieved in mature T and B lymphocytes.1–3 While tolerance is regarded as a property of antigen-reactive lymphocytes, the microenvironment in which lymphocytes first encounter antigen, including the cells which process and present antigen to T lymphocytes during immune induction, plays a key role in determining whether tolerance or sensitization will take place. For example, application of the highly reactive hapten 2,4-dinitro-1-fluorobenzene (DNFB) to the skin results in the induction of contact hypersensitivity (CH), and the sensitizing process is initiated by cutaneous antigen-presenting cells (APC), chiefly Langerhans’ cells, which carry the hapten to draining lymph nodes for presentation to T cells.4 If, however, the cutaneous surface is exposed to acute low-dose ultraviolet-B radiation (UVR) prior to application of DNFB, then sensitization fails, and the failure has been traced to a functional deficit in cutaneous APC.5 Moreover, animals first exposed to DNFB via UVR-exposed skin acquire hapten-specific tolerance6,7 and there is suspicion that the tolerance-conferring stimulus is provided by aberrant hapten-bearing APC.
Another example of unresponsiveness dictated by functionally distinct APC is the phenomenon of anterior chamber-associated immune deviation (ACAID).8–12 When APC from the iris and ciliary body (I/CB) of the eye,13–15 as well as peritoneal exudate cells (PEC) treated in vitro with transforming growth factor-β (TGF-β),16 are pulsed in vitro with a soluble protein antigen and then injected into naive, syngeneic mice, the recipients are rendered specifically unable to acquire delayed-type hypersensitivity (DTH) to the same antigen. More specifically, mice that receive ovalbumin-pulsed APC of this type cannot be induced subsequently to develop DTH to ovalbumin when they receive a subcutaneous injection of ovalbumin in complete Freund’s adjuvant (CFA). The lack of DTH in these mice is directly attributable to the effects of TGF-β on the functional properties of APC.
To discover the mechanism(s) by which TGF-β-treated APC promote T-cell unresponsiveness, we have used two macrophage (Mφ) hybridomas.17,18 In the original description, Mφ hybridoma #59 was derivatized with either nitrophenol (NP) or trinitrophenol (TNP) and was found to induce antigen-specific CH when injected into naive mice. By contrast, Mφ hybridoma #63, when derivatized with either NP or TNP, induced hapten-specific unresponsiveness in naive mice.19 More recently, our laboratory has used these hybridomas to explore the cellular basis for ACAID. The latter phenomenon is a component of ocular immune privilege, and is defined as a selective deficit in antigen-specific delayed hypersensitivity with preservation of cytotoxic T-cell responses, and enhancement of antibody responses to the same antigens. Hybridoma #59 induced immune deviation similar to ACAID in naive mice when the cells were first pulsed with bovine serum albumin (BSA) in the presence of I/CB supernatant. TGF-β2 is a major constituent of I/CB supernatant as well as aqueous humor (AH). However, when hybridoma #59 was pulsed with BSA in the absence of I/CB supernatant or TGF-β, #59 cells had no effect on subsequent immunization with BSA/CFA which induced vigorous DTH.20 By contrast, BSA-pulsed hybridoma #63 induced an ACAID-like response constitutively, as evidenced by their ability to suppress BSA-specific DTH in the absence of prior incubation with TGF-β. DNFB-derivatized #63 cells induced antigen-specific unresponsiveness, as did DNFB-derivatized #59 cells treated with TGF-β. Under the latter conditions, both Mφ hybridomas induced unresponsiveness in naive animals as well as in animals that were pre-sensitized to hapten. Production of tumour necrosis factor-α (TNF-α) by the APC was found to be responsible for tolerance induction.
MATERIALS AND METHODS
Animals
Adult BALB/c mice (8–12 weeks old) were obtained from our domestic breeding facility or were purchased from Taconic Farms (Germantown, NY). All animal procedures were approved by our Institutional Animal Care and Use Committee. Experimental procedures were carried out with animals under general anaesthesia achieved by intraperitoneal (i.p.) injection of 150 ml of a 1:1:4 mixture of ketamine 100 mg/ml stock concentration (Fort Dodge Laboratories, Inc., Fort Dodge, IA), xylazine 20 mg/ml stock concentration (Phoenix Pharmaceutical Inc., St. Joseph, MO) and 1× phosphate-buffered saline, (PBS; BioWhittaker, Inc., Walkersville, MD). Each control or experimental panel consisted of five mice and each experiment was repeated at least twice.
Reagents
DNFB and oxazolone (4-ethoxymethylene-2-phenyloxazol-5-one) were purchased from Sigma Chemical Co. (St. Louis, MO). Porcine TGF-β2 was purchased from R & D Systems (Minneapolis, MN).
Cells
Mφ hybridomas #59 and #63 were generated by fusion of P388D1 tumour cells (DBA/2 origin, H-2d) with adherent cells from spleens of CKB (C3H-derived, H-2k) mice as reported previously.17,21 Cells were maintained in complete medium containing RPMI-1640 (with penicillin, streptomycin, l-glutamate, 25 mm HEPES), 1% non-essential amino acids, 1% sodium pyruvate from BioWhittaker, Inc. (Walkersville, MD), 10% fetal bovine serum (FBS) from HyClone Laboratories, Inc. (Logan, UT), and 0·1% 2-mercaptoethanol from Sigma Chemical Co.
Preparation and derivatization of Mφ hybridomas with DNFB
Mφ hybridomas grown in complete medium were washed twice with RPMI-1640 containing penicillin, streptomycin, l-glutamate, and 25 mm HEPES and resuspended at a density of 10×106 cells/ml in RPMI-1640 after the final wash. Then, 0·5 ml of resuspended Mφ hybridomas were added to 7·0 ml induction medium and cultured overnight. The induction medium consists of RPMI-1640 with penicillin, streptomycin, l-glutamate, and 25 mm HEPES from BioWhittaker, Inc., 1% normal mouse serum from Accurate Chemical & Scientific Corp. (Westbury, NY), and 5 mg/ml BSA from Sigma Chemical Co. Cells were washed twice with RPMI-1640 after overnight culturing in induction medium and resuspended in Hanks’ balanced slat solution (HBSS) from BioWhittaker, Inc. at a density of ×106 cells/ml. Mφ hybridomas were hapten-derivatized by addition of 10 ml of 0·01% (0·8 mm) DNFB in acetone per million cells by a method described previously.22 The mixture was incubated in the dark for 30 min and vortexed every 10 min. Before use derivatized Mφ hybridomas were washed twice with RPMI-1640 and resuspended in HBSS at a concentration of 5×104 cells/ml. The Mφ hybridomas (5×103 in a 100-ml volume) were injected intravenously (i.v.) into the tail vein or in two 50 ml portions in both hindfoot pads according to protocol.
Induction and assay for tolerance or CH
Twenty-five millilitres of a 0·5%/volume solution of DNFB in acetone (185 mg) was applied to the dry-shaved abdominal, cutaneous skin of mice on the day specified in the protocol. CH was elicited 5 days after the last performed procedure by challenging one ear of each mouse with 20 ml of a 0·05%/volume solution of DNFB in acetone (15 mg). Ear swelling was measured with an engineer’s micrometre (Mitutoyo, Tokyo, Japan) 24 and 48 hr following the challenge. Ear swelling measurements are expressed as the difference in ear swelling between treated and untreated ears before and after challenge. Sensitization with oxazolone was performed analogous to that with DNFB. However, a 2-mg/ml solution of oxazolone in acetone was used and a total of 25 ml (500 mg) was applied to the dry-shaved skin of mice. Ear challenging with oxazolone was performed by applying 20 ml of a 0·5-mg/ml solution (100 mg) to the ear. Ear swelling was measured after 24 and 48 hr.
In vivo antibody treatment
Neutralizing rabbit anti-mouse-TNF-α antibody from Genzyme (Cambridge, MA), sufficient to neutralize 2000 units of TNF-α,23 or 100 mg per mouse of rabbit anti-BSA antibody from ICN Biomedicals, Inc. (Costa Mesa, CA) were injected i.p. 6 hr before injection of Mφ hybridomas.
Assessment of TNF-α protein production
Determination of TNF-α protein production by Mφ hybridomas and RAW 264.7 cells in the presence or absence of TGF-β2 was performed on supernatant using the DuoSeT enzyme-linked immunosorbent assay (ELISA) System from Genzyme. Hamster anti-mouse TNF-α antibody was used as the capture antibody and horseradish peroxidase-conjugated goat anti-mouse TNF-α antibody for detection. Both antibodies were purchased from Genzyme.
Statistical analysis
Statistical analysis of differences between means of experimental groups was determined by analysis of variance (anova). Differences between means were considered to be significant when P < 0·05. Analyses were performed using StatViewTM 512+ (Abacus Concepts, Berkeley, CA).
Reverse transcriptase–polymerase chain reaction
Messenger RNA for reverse transcriptase–polymerase chain reaction (RT-PCR) was isolated from Mφ hybridomas using the FastTrack®2·0 Kit from Invitrogen (San Diego, CA) which utilizes oligo-dT cellulose. From isolated mRNA cDNA was batch synthesized by the random primer method using avian myeloblastosis virus (AMV) RT from Promega (Madison, WI) and aliquots of this cDNA were used for PCR analysis. All standard reagents for PCR amplification were purchased from Perkin Elmer (Foster City, CA). Fifty-millilitre PCR reactions were performed in 200-ml tubes in a GeneAmp®PCR System 2400 from Perkin Elmer using the AmpliWax®PCR system and AmpliTaq®DNA polymerase. Cycling profiles were as indicated in the figures.
Intron spanning TNF-α primers used were 5′-GAA AGC ATG ATC CGC GAC GTG GA-3′ (upstream primer) and 5′-TAC GAC GTG GGC TAC AGG CTT G-3′ (downstream primer) which yielded a 279-base pair (bp) amplicon. Intron spanning control primers for the specific amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA had the following sequences. 5′-GGT GAA GGT CGG TGT GAA CGG A-3′ (upstream primer) and 5′-TGT TAG TGG GGT CTC GCT CCT G-3′. A 245-bp amplicon is generated by these primers.
RESULTS
Tolerance induction by DNFB-derivatized APC in naive mice
In the following experiments, Mφ hybridomas #59 and #63 were derivatized with the hapten, DNFB. The cells were then washed and injected (5×103) subcutaneously (s.c.) into naive BALB/c mice. Positive control mice received no injected cells. Seven days later all mice received a sensitizing dose of DNFB applied to the shaved abdominal wall skin. Five days thereafter the ears of these mice, along with those of negative controls, were challenged with 20 ml of 0·05%/volume (15 mg) DNFB. Negative control mice received neither an injection of derivatized Mφ hybridomas, nor did they have a sensitizing dose of DNFB applied to their abdominal wall skin. Thus, these mice encountered hapten for the first time when challenged and no DTH response was expected. Ear swelling was assessed with a micrometre 24 and 48 hr later. The results of a representative experiment are shown in Fig. 1, Groups A, D, and E. Mice that first received DNFB- #59 cells displayed intense CH, comparable to that of positive controls. By contrast, mice that first received DNFB- #63 displayed ear swelling responses comparable to negative controls. These findings indicate that hapten-derivatized #63 cells possess the ability to interfere with subsequent sensitization with the same hapten, i.e. DNFB- #63 cells constitutively promoted tolerance.
Figure 1.
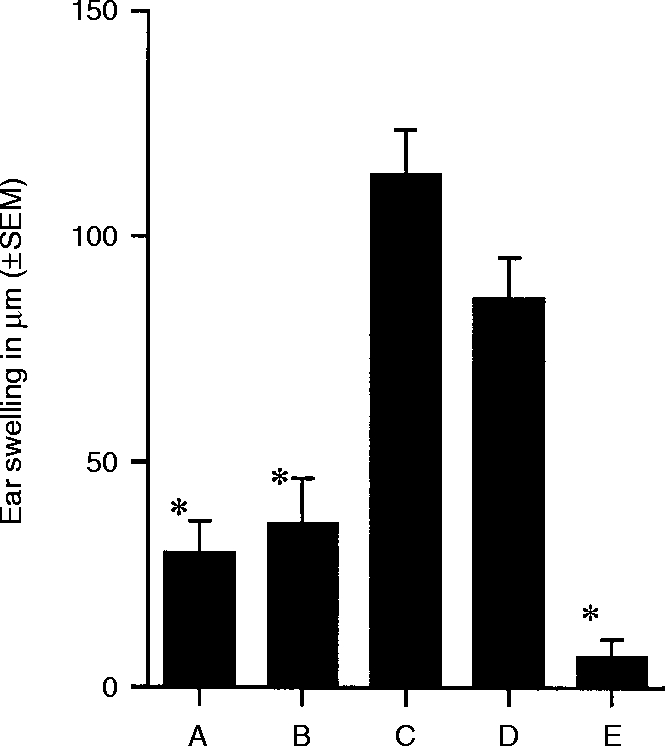
Tolerance induction by DNFB-derivatized Mφ in naive mice. Panels of five animals each received s.c. injections of 5000 DNFB-derivatized Mφ#63 (Group A), Mφ#59 treated with TGF-β2 (Group B), and Mφ#59 (Group C) into the hindfoot pads 7 days prior to sensitization with DNFB (185 mg). Positive control (Group D) and negative control (Group E) were treated as described above. All mice were ear challenged with DNFB 5 days after sensitization. CH reactions were evaluated by ear swelling measurements at 24 and 48 hr. The 24-hr ear swelling measurements are shown. * indicates that the mean value is significantly less than the positive control (P < 0·00001).
Previously, it has been shown that incubation of #59 cells with I/CB supernatant in vitro confers on the cells the capacity to suppress induction of DTH.20 This change in phenotype of Mφ hybridoma #59 has been demonstrated to depend solely on the presence of TGF-β2 (unpublished results). Thus, we included in our experiment a panel of mice that received #59 cells that were incubated with TGF-β2 over night (16–18 hr) prior to being derivatized with DNFB. The results of this experiment are presented in Fig. 1, Group B. Clearly, mice that received an injection of DNFB- #59/TGF-β2-treated cells also failed to develop CH when a sensitizing dose of this hapten was applied epicutaneously. Thus, TGF-β2 confers on #59 cells the tolerizing potential that is inherently displayed by #63 cells.
In separate experiments, DNFB-derivatized #63, #59 (untreated), and #59/TGF-β2-treated cells were injected i.v., and the responses of recipient mice were compared with those of mice that received s.c. injections of similar cells. The results were identical (data not shown); both sets of recipients that received DNFB-derivatized #63 and #59/TGF-β2-treated cells displayed unresponsiveness to DNFB, while the parallel groups that received DNFB- #59 (untreated) cells developed intense CH. These findings indicate that the route of derivatized hybridoma cell injection was irrelevant to their tolerizing capacity. We conclude that hapten-derivatized APC, can promote tolerance or sensitivity depending upon their functional status, and that TGF-β2 alters APC in a manner that enables the cells to promote unresponsiveness.
Tolerance induction by DNFB-derivatized APC in immune mice
When originally described by Kuchroo et al.19 NP-derivatized #63 hybridoma cells were able to induce unresponsiveness even when injected into mice previously sensitized to NP. Similarly, Kosiewicz et al. have demonstrated that ACAID can be induced in presensitized mice.24 In the Kosiewicz experiments, impaired DTH was achieved in ovalbumin-immune mice following i.v. injection of PEC that had been pulsed with ovalbumin in the presence of TGF-β2in vitro. We next inquired whether DNFB-derivatized hybridoma cells –#63, #59, and #59 after TGF-β2 treatment – were able to suppress DNFB-specific CH in preimmune mice. Panels of BALB/c mice were sensitized by epicutaneous application of a sensitizing dose of DNFB. Seven days later, groups of mice received subcutaneously (Group A) DNFB- #63 cells (Group B) DNFB- #59 cells, or (Group C) DNFB- #59/TGF-β2-treated cells. One week thereafter the ears of these mice, plus negative controls, were challenged with dilute DNFB. The results of a representative experiment are presented in Fig. 2. Mice that received DNFB- #63 cells or DNFB- #59/TGF-β2-treated cells displayed little or no ear swelling, whereas mice that received DNFB- #59 cells (untreated) mounted CH responses comparable to positive controls. These results indicate that the functional phenotypes of #63 and #59/TGF-β2-treated cells are similar in that both can impose unresponsiveness on animals previously sensitized to the hapten in question.
Figure 2.
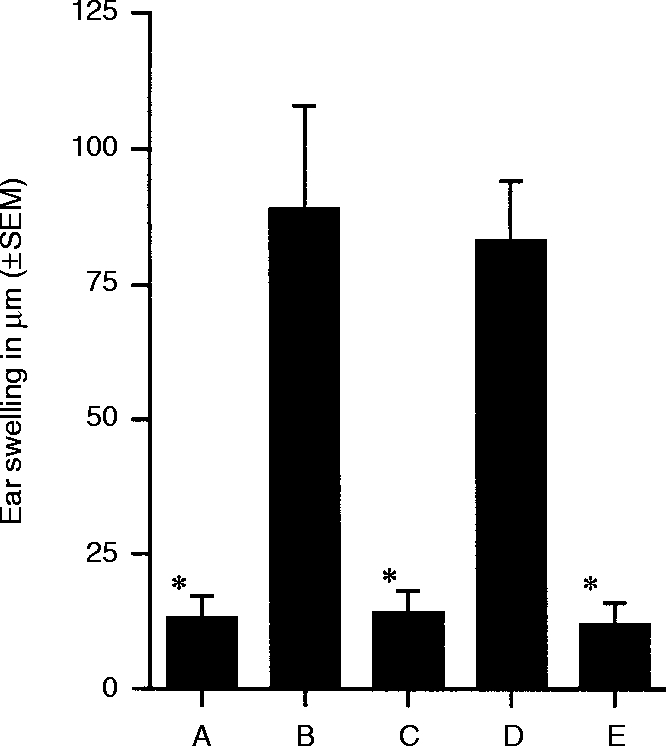
Tolerance induction by DNFB-derivatized Mφ in presensitized mice. All groups of animals but the negative control (Group E) were sensitized epicutaneously with DNFB. Seven days after sensitization animals from Group A received injections of 5000 DNFB-derivatized Mφ#63. Groups B and C received injections of 5000 Mφ#59 and Mφ#59 treated with TGF-β2, respectively. Groups D and E did not receive any treatment at this time. All groups of mice were ear challenged with DNFB 5 days after injection of Mφ. CH reactions were evaluated by ear swelling measurements at 24 and 48 hr. The 24-hr ear swelling measurements are shown. * indicates that the mean value is significantly less than the positive control (P < 0·00005).
Specific tolerance induction by DNFB- #59/TGF-β2-treated cells
DNFB and oxazolone are structurally and antigenically unrelated haptens. The next experiments examined whether the unresponsiveness achieved by DNFB- #59/TGF-β2-treated cells was hapten-specific. Panels of BALB/c mice received 5×103 DNFB- #59/TGF-β2-treated cells s.c. One week later the mice received an epicutaneous application of either DNFB or oxazolone. Within 1 week the ears of the mice were challenged with dilute solutions of the hapten used for epicutaneous application. As the results presented in Fig. 3 indicate, both positive controls displayed CH (Groups A and C) compared to their respective negative controls (Groups B and D). Mice that received DNFB- #59/TGF-β2-treated cells prior to sensitization with DNFB displayed very weak CH responses (Group E) whereas mice that received the same cells initially, followed by sensitization with oxazolone displayed intense CH when challenged with oxazolone (Group F). Thus, the unresponsiveness induced by DNFB- #59/TGF-β2-treated cells is hapten-specific.
Figure 3.
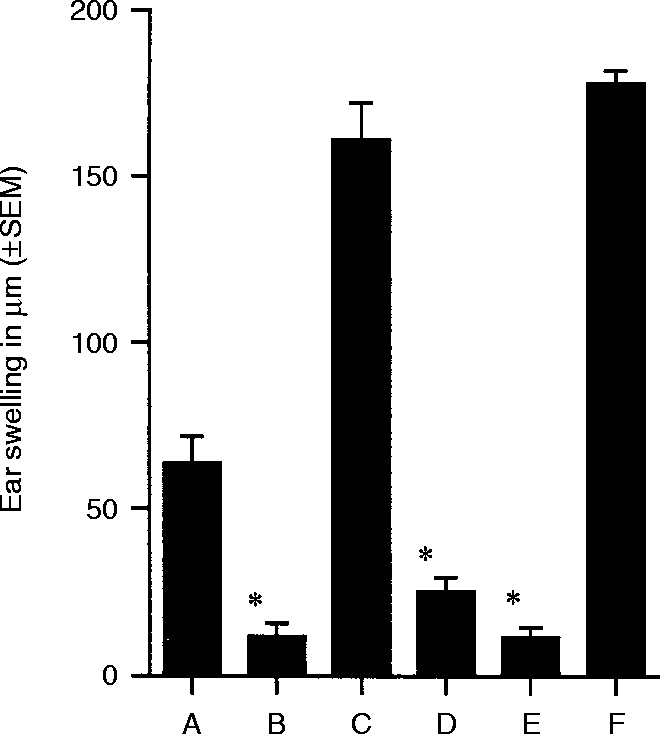
Antigen specificity of unresponsiveness to DNFB – single challenge experiments. Positive controls for CH to DNFB (Group A) or oxazolone (Group C) were sensitized epicutaneously with the appropriate hapten and challenged with the same hapten 12 days later. Negative controls for CH to DNFB (Group B) or oxazolone (Group D) encountered hapten for the first time during ear challenge. Animals in Group E were sensitized epicutaneously with 185 mg DNFB followed 7 days later by s.c. injection of 5000 DNP-Mφ#59 treated with TGF-β2. Animals in Group E were ear challenged with DNFB 12 days after sensitization. Group F mice were sensitized epicutaneously with oxazolone (500 mg), followed 7 days later by injection of 5000 DNP-Mφ#59 treated with TGF-β2, and challenged with oxazolone 12 days after sensitization. Ear swelling was measured 24 and 48 hr after challenge, 24-hr ear swelling measurements are shown. Mean values significantly less than the positive control (P < 0·005) are marked by *.
The hapten-specificity of the unresponsiveness induced by DNFB- #59/TGF-β2-treated cells was further tested by re-immunizing panels A, C, and F of the previous experiment (Fig. 3) with sensitizing doses of DNFB painted on previously untreated body wall skin 21 days after the initial epicutaneous application of hapten. The results are presented in Fig. 4. Positive controls, sensitized to DNFB 3 weeks previously (Group A, Fig. 3) mounted intense CH responses (Group A, Fig. 4), compared to new negative controls (Group D, Fig. 4). Mice originally sensitized with oxazolone (Group C, Fig. 3) were readily sensitized by the application of DNFB 21 days later (Group C, Fig. 4), but mice that had received DNFB- #59/TGF-β2-treated cells (Group F, Fig. 3), while able to mount CH to oxazolone in the previous experiment, failed to acquire CH following sensitization with DNFB (Group B, Fig. 4). These results indicate that sensitization with oxazolone has no effect on the ability of mice to become sensitized subsequently to DNFB. In addition, the findings indicate that the unresponsiveness induced by DNFB- #59/TGF-β2-treated cells is long-lasting – at least through the 28-day interval of this particular experiment.
Figure 4.
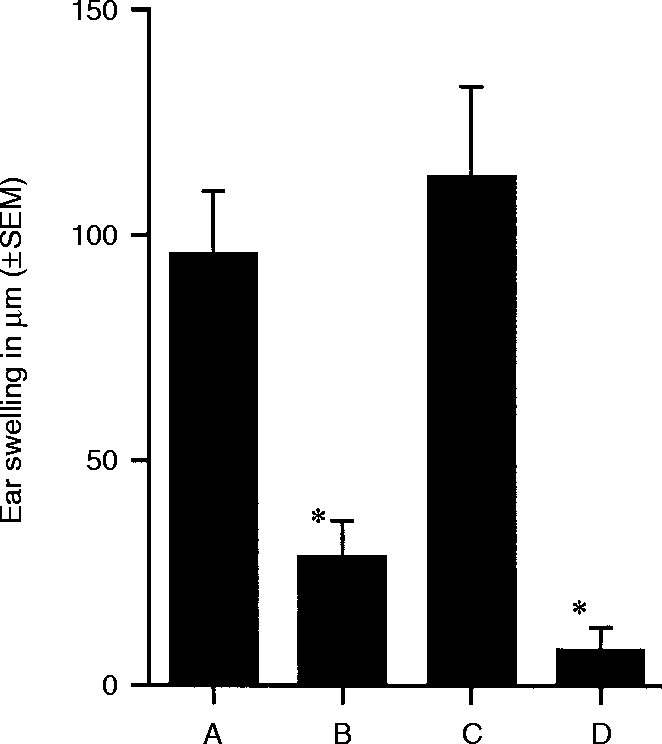
Antigen specificity of unresponsiveness to DNFB – double challenge experiments. Mice sensitized to oxazolone (Fig. 3, Group C) and mice that received 5000 DNP-Mφ#59 treated with TGF-β2 7 days after epicutaneous sensitization with oxazolone (Fig. 3, Group F) were sensitized epicutaneously with DNFB 21 days after initial sensitization with oxazolone, Groups A and B, respectively. Positive control animals (Group C) were sensitized epicutaneously with DNFB, received a booster sensitization 21 days later, and were ear challenged with DNFB 5 days after the last sensitization. Negative control animals (Group D) encountered DNFB for the first time during ear challenge. Ear swelling was measured at 24 and 48 hr after challenge. The 48-hr ear swelling measurements are shown. Mean values that are significantly less than the positive control (P < 0·005) are marked by *.
Identification of molecules important in tolerance induction
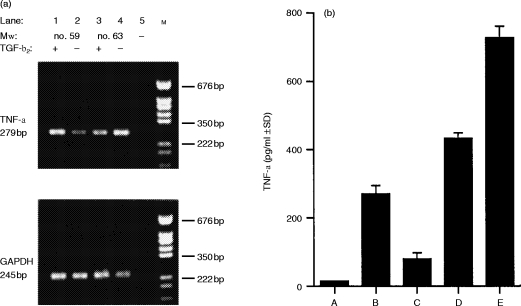
Cytokines released by APC, as well as cell surface molecules expressed by these cells, have been implicated (positively or negatively) in tolerance induction in other systems. Specifically, interleukin-10 (IL-10), TNF-α and Fas ligand, under certain conditions, have been identified to promote unresponsiveness,12,21,25–30 whereas interferon-γ (IFN-γ) and IL-1231–34 have been found to promote sensitization. Untreated #59 and #63 as well as #59 and #63 cells treated with TGF-β2 were subjected to mRNA analysis by RT-PCR using primers specific for messages of the molecules mentioned above. Whether the cells were untreated, or treated with TGF-β2, mRNA for IL-10, Fas ligand, IL-12, or IFN-γ (data not shown) were not detected. However, mRNA was detected for TNF-α. As Fig. 5(a) reveals, message for TNF-α was detected at high levels by RT-PCR in mRNA isolated from #59 cells that had been treated with TGF-β2 as well as from #63 cells whether they had been treated with TGF-β2 or not. However, untreated #59 cells, the only APC not able to induce unresponsiveness under the given conditions, showed low levels of TNF-α mRNA as compared to those Mφ hybridomas with tolerance/unresponsiveness-inducing capacity. Findings from our RT-PCR analysis are reflected in the ability of the Mφ hybridomas to secrete TNF-α protein. As shown in Fig. 5(b), untreated Mφ hybridomas #59, which are not capable of inducing antigen-specific unresponsiveness to hapten, secrete virtually no TNF-α protein. However, after treatment with TGF-β2 Mφ hybridomas #59 clearly up-regulate TNF-α protein production. While Mφ hybridomas #63 up-regulate TNF-α protein production after TGF-β2 treatment, these cells also produce constitutively detectable levels of TNF-α protein. Overall, TNF-α protein production of tolerogenic APC is clearly below inflammatory levels of the cytokine as seen in comparison with the production of TNF-α protein by untreated RAW 264.7 cells.
Figure 5.
Assessment of TNF-α mRNA and protein production. (a) Messenger RNA isolate from Mφ#59 and #63 cultured in the presence or absence of TGF-β2 was subjected to reverse transcription followed by touchdown PCR (70–55° at one cycle per 0·5° and four additional cycles at 55°) with primers specific for TNF-α and GAPDH. Lane 1, Mφ#59 treated with TGF-β2; lane 2, Mφ#59; lane 3, Mφ#63 treated with TGF-β2; lane 4, Mφ#63; lane 5, control PCR reaction performed on mock reverse transcribed mRNA (without RT enzyme); lane M, pGEM(DNA marker from Promega (Madison, WI). (b) TNF-α secreted by Mφ#59, #69 and RAW 264.7 cells cultured in induction medium was determined by ELISA. Group A, Mφ#59; Group B, Mφ#59 treated with TGF-β2; Group C, Mφ#63; Group D, Mφ#63 treated with TGF-β2; Group E, untreated RAW 264.7 cells.
Effect of neutralizing anti-TNF-α antibody on the ability of DNFB- #63 cells to induce unresponsiveness
Although TNF-α is usually regarded as a pro-inflammatory cytokine,35–37 it has been demonstrated to be a key player in the hapten-specific unresponsiveness that develops when a hapten is painted on skin previously exposed to UVR.23,26,37 Therefore, increased levels of TNF-α mRNA in tolerance-inducing Mφ hybridomas, coupled with up-regulation of TNF-α protein secretion suggested that the ability of these cells to promote unresponsiveness might be related to their production of this cytokine. To test this possibility, a neutralizing rabbit anti-murine TNF-α antibody was injected i.p. into one panel of DNFB-sensitized BALB/c mice (Group A). Another panel of DNFB-sensitized BALB/c mice (Group B) received an i.p. injection of the irrelevant rabbit antibody, anti-BSA. Six hours later, the mice received s.c. injections of 5×103 DNFB/#63 cells. The ears of mice were challenged 5 days later (Fig. 6). Mice that were treated with anti-BSA antibody followed by injection of DNFB/#63 cells failed to acquire CH (Group B). By contrast, mice that had received anti-TNF-α antibody prior to injection of DNFB/#63 cells were fully able to develop CH (Group A). These findings indicate that the TNF-α produced constitutively by hapten-derivatized- #63 cells is responsible for the ability of these cells to suppress sensitization to the hapten used for derivatization.
Figure 6.
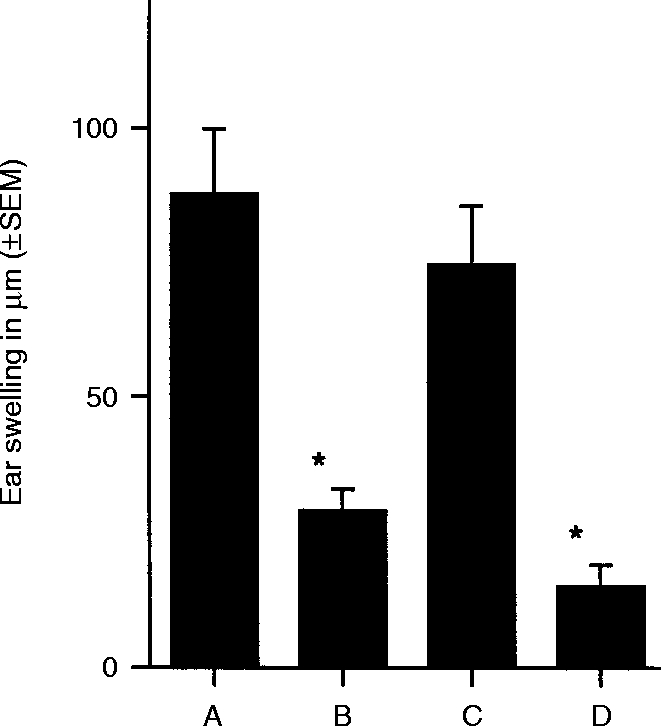
Abrogation of unresponsiveness in presensitized mice by neutralizing antibody specific for TNF-α. All groups of mice but the negative control (Group D) were sensitized with DNFB on day 0. Animals in Group A received an i.p. injection of neutralizing anti-TNF-α antibody 6 hr before injection of DNP-Mφ#63. Control animals (Group B) were injected with anti-BSA antibody 6 hr before injection of DNP-Mφ#63. Group C, positive control for CH, and Group D, negative control group, did not receive antibody injections nor did they receive injections of Mφ. All groups of animals were ear challenged with DNFB and ear swelling was assessed 24 and 48 hr after challenge, only 24 hr measurements are shown. * indicates that the mean value is significantly less than the positive control (P < 0·005).
DISCUSSION
The goal of achieving immunological tolerance at will has been a prominent activity of immunologists for more than 50 years. For most of the time, the focus of research has been on those cells of the immune system that express recognition structures for antigen: T cells and B cells. Experimental strategies can generate T and/or B cells that participate in antigen-specific tolerance – passively (by deletion or anergy) or actively (by immune deviation or suppression).1–3,9,38–40 However, it has been difficult to translate these strategies into clinical practice, whether to prevent immune rejection of allografts, to ameliorate autoimmune disease, or to lessen the morbidity of agents that cause cutaneous hypersensitivity reactions of the contact dermatitis type.
APC in lymphocyte activation have become increasingly appreciated and the possibility that an important pathway to immune tolerance may lead through APC is gaining credibility. The recent, controversial proposal41 that the immune system is motivated by ‘danger’ signals, rather than the classical self/non-self paradigm, addresses the importance of APC in moulding the qualities of the immune response to antigens. In this proposal, APC located in peripheral tissues such as skin are postulated to be the first detectors of ‘danger.’ It is in this context that the results reported here find their relevance.
Mφ hybridoma #59 displays antigen-presenting properties of a conventional APC.When #59 cells were derivatized with the hapten, DNFB, and injected intracutaneously into naive mice, hapten-specific CH was induced. By contrast, Mφ hybridoma #63 failed to function as a conventional APC; when derivatized with DNFB and injected into naive mice, #63 cells failed to induce CH. Moreover, when #59 cells were first exposed in vitro to TGF-β2 and then hapten-derivatized, the cells lost their capacity to induce CH. Instead, DNFB-derivatized, TGF-β2-treated #59 cells induced hapten-specific unresponsiveness. The capacity of TGF-β2-treated #59 cells to promote tolerance was mirrored by untreated #63. These findings are remarkable on at least two accounts. First, the data suggest that the phenotype of the immune response to DNFB can be manipulated by influencing the functional properties of the cells that present antigen to naive T cells. In this circumstance, it is the APC which dictates the immune outcome, not the responding T cell. Second, these results indicate that the functional properties of APC can be dramatically altered by the pleiotropic cytokine TGF-β2.
APC appear to be functionally plastic, driven at least in part by cytokines in their microenvironment. There is now a growing list of cytokines that have the capacity to shape the functional properties of APC. It has been known for many years that IFN-γ can bias APC function in the direction of immunity mediated by T cells of the T helper type 1 (Th1) type.32,37 More recently, IL-10 has been implicated in dictating APC function, typically in the direction of promoting the activation of Th2-type cells.12,25,32,42,43 TNF-α is a third cytokine for which experimental evidence suggests an important role in dictating APC function.23,35–37 Based in part on the findings reported here, TGF-β must be included on this list. It has long been implicated in the phenomena of (a) anterior chamber associated immune deviation,17,44 and (b) oral tolerance.39 TGF-β2 is constitutively present in AH of the anterior chamber,45 and conventional APC treated in vitro with AH acquire the capacity to induce immune deviation in vivo.16 Moreover, neutralization of TGF-β within AH robs the fluid of this property. In oral tolerance, the regulatory T cells that can adoptively transfer the unresponsive state secrete a unique spectrum of cytokines, including TGF-β2.39
The mechanism(s) by which TGF-β2 converts conventional APC into cells that induce immune deviation remain unclear. Thioglycollate-induced PEC cultured overnight secrete large amounts of IL-12.46–48 Consequently, if overnight cultured PEC are pulsed with ovalbumin and are then used to stimulate ovalbumin-specific T cells in vitro, the responding cells acquire a Th1-like phenotype – secreting IFN-γ and IL-2.46 On the other hand, if PEC are first treated with TGF-β2 and then cultured overnight, such cells secrete much less IL-12 than their untreated counterparts and secrete significant amounts of TGF-β2.47 Despite these changes in cytokine production, TGF-β2-treated PEC can still present peptides of ovalbumin to CD4+ ovalbumin-specific T cells. Although the responding T cells proliferate, they produce IL-4, rather than IFN-γ and IL-2, implying a shift to the Th2-phenotype. We have examined the two cell lines for mRNA of pertinent accessory signals: IL-10, IL-12, Fas ligand, IFN-γ and TNF-α. Only mRNA for TNF-α was detectable in #59 and #63. The #63 cells constitutively contained much higher levels of TNF-α mRNA than did #59 cells and treatment of #59 cells with TGF-β2 enhanced TNF-α mRNA levels – to levels rivalling #63. This observation was paralleled by the secretion of TNF-α. Thus, mRNA levels determined by RT-PCR and amounts of secreted TNF-α protein correlated positively with the capacity of hybridoma cells to induce immune deviation in vivo. The #59 cells which induced CH had low constitutive levels of TNF-α mRNA and produced TNF-α protein at the detection limit of the assay; #63 cells, and TGF-β2-treated #59 cells, both of which induced immune deviation, displayed higher levels of TNF-α mRNA and secreted the cytokine.
The observation that neutralizing anti-TNF-α antibody prevented hapten-derivatized #63 cells from inducing immune deviation in vivo is of considerable interest. TNF-α is a Janus-like cytokine with a multitude of functions. Of particular interest here is that TNF-α has been demonstrated to alter the functional properties of epidermal Langerhans’ cells and dermal dendritic cells in a negative way, robbing the cells of the capacity to present epicutaneously applied hapten in a fashion that leads to CH.23,49 We propose that the negative influence of TNF-α on Langerhans’ cell function is relevant to their role in promoting immune deviation adopted by Mφ hybridoma cells. Recent evidence indicates that intradermal injection of TNF-α alters the cytoskeleton of epidermal Langerhans’ cells and interferes transiently with the ability of the cells to escape from the skin following epicutaneous application of hapten.5 Moreover, hapten-derivatized Langerhans’ cells that are first incubated with TNF-α fail to induce CH when injected intracutaneously into naive, syngeneic mice.42 Similarly, addition of exogenous TNF-α to mixed lymphocyte cultures markedly reduces the resultant T-cell proliferation.28,50
In the aggregate, these observations suggest APC bathed in a microenvironment containing TNF-α are inefficient at activating T cells that generate conventional (Th1-like) immunity. The findings described further indicate that TNF-α-influenced APC actually go one step further and have the potential to promote immune deviation. A similar conclusion was drawn by Ferguson and colleagues who treated mice with anti-TNF-α antibody and prevented the induction of immune deviation following injection of antigen into the AC of the eye.27 Our results indicate that TGF-β2 induces the Mφ hybridomas to produce subinflammatory amounts of TNF-α which are biologically significant and involved in the induction of a form of tolerance that resembles ACAID.
The capacity of hapten-derivatized #63 or TGF-β2-treated #59 cells to prevent expression of CH in mice sensitized to DNFB warrants comment. Inducing unresponsiveness has traditionally been more difficult in sensitized animals and most attempts at tolerance induction in this setting have failed. There are, however, a few exceptions. With large oral doses of certain antigens, immunity can be overcome, and oral tolerance can be induced.39 This strategy has been used in experimental allergic encephalomyelitis, which is thought to be mediated by Th1-type cells. Kosiewicz et al.24,51 demonstrated that injection of ovalbumin into the AC of the eye of mice immunized with ovalbumin abolished the ovalbumin-specific DTH response. A similar outcome was observed if PEC were treated with TGF-β2, pulsed with ovalbumin, and then injected i.v. into ovalbumin-immune recipients. Our findings strongly resemble this last experimental observation – with the exception that route of administration proved to be unimportant.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AR44130, R37 EY05678, and F32 EY06731. We thank Dr Jie Zhang-Hoover for performing TNF-α ELISA assays.
Glossary
Abbreviations
- Ab
antibody
- AC
anterior chamber
- ACAID
anterior chamber-associated immune deviation
- AH
aqueous humor
- AMV RT
avian myeloblastosis virus reverse transcriptase
- APC
antigen-presenting cell
- CH
contact hypersensitivity
- DNFB
2,4-dinitro-1-fluorobenzene
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- I/CB
iris/ciliary body
- Mφ
macrophage
- RT
reverse transcriptase
- TGF-β2
transforming growth factor-β2
- TNF-α
tumour necrosis factor-α
- UVR
ultraviolet B radiation
REFERENCES
- 1.Chiller JM, Habicht GS. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971;171:813. [PubMed] [Google Scholar]
- 2.Murphy DB. T cell mediated immunosuppression. Curr Opin Immunol. 1993;5:411. doi: 10.1016/0952-7915(93)90061-v. [DOI] [PubMed] [Google Scholar]
- 3.Holan V, Sedlackova K, Ruzickova M. Production of high levels of Th1 and Th2 cytokines in mice with acquired transplantation tolerance. Cell Immunol. 1996;174:7. doi: 10.1006/cimm.1996.0287. [DOI] [PubMed] [Google Scholar]
- 4.Streilein JW. The Next Generation in the Skin Immune System (SIS) Boca Raton, Florida: CRC Press, Inc.; 1990. Skin associated lymphoid tissues (SALT) p. 26. [Google Scholar]
- 5.Bacci S, Nakamura T, Streilein JW. Failed antigen presentation after UVB radiation correlates with modifications of Langerhans cell cytoskeleton. J Invest Dermatol. 1996;107:838. doi: 10.1111/1523-1747.ep12330994. [DOI] [PubMed] [Google Scholar]
- 6.Kripke ML. Immunological unresponsiveness induced by ultraviolet radiation. Immunol Rev. 1984;80:87. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 7.Kripke ML. Immunological effects of ultraviolet radiation. J Dermatol. 1991;18:429. doi: 10.1111/j.1346-8138.1991.tb03111.x. [DOI] [PubMed] [Google Scholar]
- 8.Niederkorn JY, Mayhew E. Role of splenic B cells in the immune privilege of the anterior chamber of the eye. Eur J Immunol. 1995;25:2783. doi: 10.1002/eji.1830251011. [DOI] [PubMed] [Google Scholar]
- 9.Streilein JW, Ksander BR, Taylor AW. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557. [PubMed] [Google Scholar]
- 10.Niederkorn JY, Ferguson TA. Anterior chamber associated immune deviation (ACAID) In: Pepose J, Holland G, Wilhelmus K, editors. Ocular Infections and Immunity. St. Louis: Mosby – Year Book, Inc.; 1996. p. 96. chap. 7. [Google Scholar]
- 11.Streilein JW. Immune regulation and the eye: a dangerous compromise. FASEB J. 1987;1:199. [PubMed] [Google Scholar]
- 12.D’Orazio TJ, Niederkorn JY. A novel role for TGF-β and IL-10 in the induction of immune privilege. J Immunol. 1998;160:2089. [PubMed] [Google Scholar]
- 13.Hara Y, Okamoto S, Rouse B, Streilein JW. Evidence that peritoneal exudate cells cultured with eye-derived fluids are the proximate antigen-presenting cells in immune deviation of ocular type. J Immunol. 1993;151:5162. [PubMed] [Google Scholar]
- 14.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID) II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146:3018. [PubMed] [Google Scholar]
- 15.Williamson JS.P, Bradley D, Streilein JW. Immunoregulatory properties of bone marrow-derived cells in the iris and ciliary body. Immunology. 1989;67:96. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID) III. Induction of ACAID depends upon intraocular transforming growth factor-β. Eur J Immunol. 1992;22:165. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- 17.Uchida T, Ju S-T, Fay A, Liu Y-N, Dorf ME. Functional analysis of macrophage hybridomas. I. Production and initial characterization. J Immunol. 1985;134:722. [PubMed] [Google Scholar]
- 18.Ju S-T, Dorf ME. Functional analysis of cloned macrophage hybridomas IV. Induction and inhibition of mixed lymphocyte responses. J Immunol. 1985;134:3722. [PubMed] [Google Scholar]
- 19.Kuchroo VK, Minami M, Diamond B, Dorf ME. Functional analysis of cloned macrophage hybridomas. VI. Differential ability to induce immunity or suppression. J Immunol. 1988;141:10. [PubMed] [Google Scholar]
- 20.Hara Y, Caspi RR, Wiggert B, Dorf M, Streilein JW. Analysis of an in vitro-generated signal that induces systemic immune deviation similar to that elicited by antigen injected into the anterior chamber of the eye. J Immunol. 1992;149:1531. [PubMed] [Google Scholar]
- 21.Kawasaki H, Martin CA, Uchida T, et al. Functional analysis of cloned macrophage hybridomas. V. Induction of suppresser T cell responses. J Immunol. 1986;137:2145. [PubMed] [Google Scholar]
- 22.Dai R, Grammer SF, Streilein JW. Fresh and cultured Langerhans cells display differential capacities to activate hapten-specific cells. J Immunol. 1993;150:59. [PubMed] [Google Scholar]
- 23.Yoshikawa T, Streilein JW. Genetic basis of the effects of ultraviolet light B on cutaneous immunity. Evidence that polymorphism at the TNF-α and Lps loci governs susceptibility. Immunogenetics. 1990;32:398. doi: 10.1007/BF00241633. [DOI] [PubMed] [Google Scholar]
- 24.Kosiewicz MM, Okamoto S, Miki S, Ksander BR, Shimizu T, Streilein JW. Imposing deviant immunity on the presensitized state. J Immunol. 1994;153:2962. [PubMed] [Google Scholar]
- 25.Niizeki H, Streilein JW. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin is mediated via interleukin-10. J Invest Dermatol. 1997;109:25. doi: 10.1111/1523-1747.ep12276415. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu T, Streilein JW. Local and systemic consequences of acute, low-dose ultraviolet B radiation are mediated by different immune regulatory mechanisms. Eur J Immunol. 1994;24:1765. doi: 10.1002/eji.1830240807. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson T, Herndon JM, Dube P. The immune response andthe eye: a role for TNFα in anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 1994;35:2643. [PubMed] [Google Scholar]
- 28.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 30.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 31.Dipiro JT. Cytokine networks with infection: mycobacterial infections, leishmaniasis, human immunodeficiency virus infection, and sepsis. Pharmacotherapy. 1997;17:205. [PubMed] [Google Scholar]
- 32.Trinchierie G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 33.Dewaal MR. The role of type I interferons in the differentiation and function of Th1 and Th2 cells. Semin Oncol. 1997;24(Suppl. 9):S9. [PubMed] [Google Scholar]
- 34.Gorham JD, Guler ML, Murphy KM. Genetic control of interleukin 12 responsiveness: implications for disease pathogenesis. J Mol Med. 1997;75:502. doi: 10.1007/s001090050135. [DOI] [PubMed] [Google Scholar]
- 35.Wallace JL, Chin BC. Inflammatory mediators in gastrointestinal defense and injury. Proc Soc Exp Biol Med. 1997;214:192. doi: 10.3181/00379727-214-44087. [DOI] [PubMed] [Google Scholar]
- 36.Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G. TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol. 1997;72:137. doi: 10.1016/s0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 37.Constant SL, Bottomly K. Induction of Th1 and Th2, CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 38.Streilein JW. Immunological non-responsiveness and acquisition of tolerance in relation to immune privilege in the eye. Eye. 1995;9:236. doi: 10.1038/eye.1995.46. [DOI] [PubMed] [Google Scholar]
- 39.Weiner HL, Friedman A, Miller A, et al. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 40.Parker DC, Eynon EE. Antigen presentation in acquired immunological tolerance. FASEB J. 1991;5:2777. doi: 10.1096/fasebj.5.13.1916102. [DOI] [PubMed] [Google Scholar]
- 41.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 42.Dai R, Streilein JW. Ultraviolet B-exposed and soluble factor-pre-incububated epidermal Langerhans cells fail to induce contact hypersensitivity and promote DNP-specific tolerance. J Invest Dermatol. 1997;108:721. doi: 10.1111/1523-1747.ep12292099. [DOI] [PubMed] [Google Scholar]
- 43.Kurimoto I, Van Rooijen N, Dijkstra CD, Streilein JW. Role of phagocytic macrophages in induction of contact hypersensitivity and tolerance by hapten applied to normal and ultraviolet B-irradiated skin. Immunology. 1994;82:281. [PMC free article] [PubMed] [Google Scholar]
- 44.Wilbanks GA, Streilein JW. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-β. Eur J Immunol. 1992;22:1031. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- 45.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthal Vis Sci. 1991;32:2201. [PubMed] [Google Scholar]
- 46.Takeuchi M, Kosiewicz MM, Alard P, Streilein JW. On the mechanism by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur J Immunol. 1997;27:1648. doi: 10.1002/eji.1830270709. [DOI] [PubMed] [Google Scholar]
- 47.Simpson AE, Tomkins PT, Cooper KL. An investigation of the temporal induction of cytokine mRNAs in LPS-challenged thioglycollate-elicited murine peritoneal macrophages using the reverse transcription polymerase chain reaction. Inflam Res. 1997;46:65. doi: 10.1007/s000110050078. [DOI] [PubMed] [Google Scholar]
- 48.Zhang T, Kawakami K, Qureshi MH, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincek V, Kurimoto I, Medema J-P, Prieto E, Streilein JW. TNF-alpha polymorphism correlates with deleterious effects of ultraviolet B light on cutaneous immunity. Cancer Res. 1993;53:728. [PubMed] [Google Scholar]
- 50.Gao JX, Madrenas J, Zeng W, Zhong R, Grant D. Generation of dendritic cell-like antigen-presenting cells in long-term mixed leucocyte culture: phenotypic and functional studies. Immunology. 1997;91:135. doi: 10.1046/j.1365-2567.1997.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosiewicz MM, Streilein JW. Intraocular injection of class II-restricted peptide induces an unexpected population of CD8 regulatory cells. J Immunol. 1996;157:1905. [PubMed] [Google Scholar]