Abstract
Mycobacterium leprae-specific immunoglobulin G1 (IgG1) antibodies in patients with leprosy show a direct correlation with bacterial load (ρ=0·748; P < 0002) suggesting that IgG1 B-cell responses may be surrogate markers of disease progression. To investigate if this upregulation was a general feature of IgG1 responses to all M. leprae (ML) antigens, we analysed responses to several recombinant purified ML heat-shock proteins (HSP). Three recombinant HSPs (ML10 K, ML 18 K and ML 65 K) were tested for their ability to induce various IgG subclasses in patients with either the lepromatous (LL/BL, n = 26) or tuberculoid form (BT/TT, n = 39) of the disease as well as in healthy households (HC, n = 14) and endemic controls (EC = 19). Our major findings were: (1) selective augmentation of IgG1 antibody responses to ML10 K; (2) recognition of a restricted number of epitopes across the disease spectrum and healthy controls by IgG1 antibodies; (3) dominant recognition of cross-reactive epitopes which were common to both ML and MT 10 K. This response was not related to contamination with endotoxin. Epitope mapping using 15-mer overlapping peptides spanning the ML 10 000 MW revealed an immunodominant IgG1 binding peptide (aa41–55) in patients as well as healthy controls. This peptide is a shared epitope with M. tuberculosis 10 K suggesting that postswitched IgG1 B cells recognizing this epitope rather than naive B cells are being expanded.
INTRODUCTION
Dysregulation of both B- and T-cell responses is observed in leprosy with antibody responses being augmented towards the lepromatous or disseminated form of the disease and depression of T-cell responses.1–3 The role of antibody is not clear in either protection or disease pathogenesis in leprosy. In the murine model augmented antibody responses are associated with activation of T helper 2 (Th2) subset and progressive disease in several intracellular infections.4,5 Cytokine secreted by the Th2 subset (interleukin-4; IL-4) selectively augment immunoglobulin E (IgE) antibody responses in the murine model6 and IgG4 and IgE in parasitic infections in humans.7 Dissection of antibody responses at the isotype and IgG subclass level to whole Mycobacterium leprae sonicate in leprosy has not indicated selective activation of either of the antibody isotypes (IgG4 and IgE);8 rather we have observed a selective augmentation of M. leprae (ML)-specific IgG1 and IgG3 antibodies towards the lepromatous pole of the disease.8 Lepromatous patients also showed polyclonal activation of B-cell responses for all isotypes and IgG subclasses.8
The current study addresses the issue of whether IgG1 upregulation in patients with the lepromatous form of the disease is a general feature of B-cell responses to all ML antigens, which may indicate a polyclonal activation-related event, or if there is selective recognition of ML antigens and epitopes by IgG1 antibodies. Among the ML proteins, heat shock proteins (HSP) have been shown to be dominant targets of immune responses in both experimental models (reviewed by Coates9) and in patients with leprosy.10 We have therefore carried out detailed analysis of IgG subclass antibody responses to this group of antigens.
MATERIALS AND METHODS
Patients and controls
Newly diagnosed leprosy patients presenting at the Marie Adelaide Leprosy Centre (MALC) were recruited to our studies and have been described in detail elsewhere.11 Patients are diagnosed clinically as well as histologically on a 4-mm punch biopsy taken from the edge of an active lesion. Sixty-five patients from across the leprosy spectrum (lepromatous form, LL/BL = 26; tuberculoid form, BT/TT = 39) who had not been treated for leprosy previously were included in the study. Healthy controls included household contacts of leprosy patients with active disease (HC = 14) and endemic controls (EC = 19) who were employees of the Aga Khan University (AKU) and had no previous history of exposure to leprosy. Ethical approval was obtained from both the AKU ethical committee and MALC Human Rights Protection Committee. Written/oral consent as appropriate was obtained from both patients and control groups.
Antigens
M. leprae 10 000 MW (ML 10 K; batch ML 10-2) and M. tuberculosis 10 000 MW (MT 10 K; batch MT10-2) 18 000 MW (ML 18 K Lot #36-38) and 65 000 MW (65 K Lot #65-5C) antigens were obtained from the World Health Organization (WHO) reference reagent bank through the courtesy of Dr Jan van Embden. The M. leprae 10 000 MW (ML 10 K) contains 603 units/mg of endotoxin and M. tuberculosis 10 000 MW (MT 10 K) contains 698 units/mg of endotoxin. M. leprae sonicate (ML) Lot CD197 was obtained through the courtesy of Dr J. Colston, Mill Hill, UK. Endotoxin (LPS Lot#103H4009)) was obtained from Sigma Chemicals, St Louis MO.
Antisera
Five millilitres of blood collected from leprosy patients was allowed to separate overnight at 4°. Serum was removed and centrifuged at 400 g for 15 min; the clear supernate was distributed in small aliquots and frozen at −70° before use.
Reagents, monoclonal antibodies and conjugates
Monoclonal antibodies specific for human IgG subclasses: HP 6001(anti-IgG1), HP 6002 (anti-IgG2), HP 6047 (anti-IgG3), HP 6023 (anti-IgG4) prepared at the Center for Disease Control, Atlanta, GA were a gift from Dr Reimer. The specificity, evaluation and performance characteristics of these antibodies are described in detail elsewhere.12,13 Goat antihuman IgG (Fc specific) and goat antimouse IgG (H+L chain specific), conjugated to alkaline phosphatase were commercially obtained (Jackson Immuno Research Laboratories, West Grove, PA) and diluted according to the manufacturer's recommendations.
Synthesiz of overlapping 15-mer peptides of ML and MT 10 000 MW
Overlapping peptides spanning the whole length of the 10 000 MW protein were synthesized by manual solid phase synthesiz (Ramps, Du Pont, Stevenage, UK) using Fmoc chemistry on Rink resin (4–2′, 4′-dimethoxyphenyl- Fmoc-aminomethyl)-phenoxy resin; Calbiochem Nottingham, UK) Fmoc-protected amino acids (Bachem, Bubendorf, Switzerland) were converted to the hydroxybenzotriazole-activated esters by treatment with hydroxybenzotriazole and N,N′-diisopropylcarbodiimide in dimethyl formamide (DMF). Subsequent coupling reactions were performed in DMF and the Fmoc groups were removed with 50% piperidine in DMF followed by a series of washes in DMF. After synthesiz, side chain protecting groups were removed and the peptides were cleaved off the resin in trifluoroacetic acid in the presence of appropriate scavengers. After cleavage, peptides were precipitated with diethylether and their purity assessed by analytical high pressure liquid chromatography (HPLC). The peptides were 15-mers overlapping by five amino acids with the exception of the C-terminal peptide (amino acids 86–100). Homologous cross-reactive peptides from MT 10 000 MW were also synthesized to span the entire MT 10 000 MW antigen. This comprised of an additional seven peptides with substitutions of one to four amino acids per peptide.
Quantitation of IgG subclasses to M. leprae recombinant antigens ML 10 K, MT 10 K, ML 18 K, ML 65 K and bacterial endotoxin (lipopolysaccharide; LPS)
IgG subclasses were quantitated using an enzyme-linked immunosorbent assay (ELISA), as previously described.8 Briefly, Immulon 4 plates were coated with 100 μl of each antigen at 1 μg/ml in carbonate buffer pH 9·6 for 2 hr at 37° and then overnight at 4°. Phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) was added for 2 hr at 37° to block free sites. One hundred microlitres of sera diluted in PBS containing 0·05% Tween 20 and 1·0% BSA were added and incubated for 2 hr at 37° and then overnight at 4°. Monoclonal antibodies specific for each of the IgG subclasses and IgE were added at saturation concentrations of 1:1000 for HP 6001, HP 6002 and HP 6047 and further incubated overnight at 4°. Alkaline phosphatase-labelled goat antimouse IgG was then added and incubated for 2 hr at 37°. The plates were finally developed with alkaline phosphatase substrate. Each incubation was followed by three washes with PBS containing 0·05% Tween 20 to remove unbound protein. All test sera were run at a minimum of three dilutions and the activity was expressed as (optical density in the linear range×dilution) of the sera. In each assay a reference pool containing high titres of IgG subclass antibodies was used as a calibrator for interassay variability (coefficient of variation <5%). To control for non-specific binding, pooled sera from normal healthy donors were used as the negative control for each assay.
IgG subclass antibodies to 10 K and its 15-mer peptides
Immulon 4 plates were coated with 0·1 μg/well of M. leprae 10 K or 20 μg/well of the individual peptides from ML or MT 10 K in carbonate buffer pH 9·6 for 2 hr at 37° and then overnight at 4°. The plates were blocked with 200 μl PBS containing 5% BSA for 2 hr at 37° and subsequently washed in PBS containing 0·05% Tween 20 (PBS–Tween) three times. One hundred microlitres of sera diluted in PBS–Tween containing 1·0% BSA were added to both peptide-coated and uncoated blocked wells (serum blank) at a single dilution of 1/20 and incubated for a further 2 hr at 37° and then overnight at 4°. For detection of IgG subclass-specific antibodies, wells were incubated with monoclonal antibodies specific for IgG1 (HP 6069) or IgG3 (HP 6047) subclasses for 2 hr at 37° and then overnight at 4°. The final incubation was with antimouse IgG conjugated to alkaline phosphatase (Fc-specific; Jackson Laboratories). A serum pool from five healthy endemic donors and IgG–10 K positive and negative sera from leprosy patients were run in each assay to control for background binding to peptides, non-specific binding caused by the presence of high titres of nonrelevant antibodies in leprosy sera and interassay variability.
RESULTS
Comparison of IgG subclass antibody responses to M. leprae recombinant antigens (ML 10 K,ML 18 K and ML 65 K)
An ELISA was developed to detect antibodies to all the recombinant antigens. All sera were titrated to obtain a readable optical density and the results are expressed as optical density multiplied by the dilution of the serum. The results are shown in Table 1. IgG antibody subclass responses were compared in patients and their household contacts to differentiate responses associated with disease compared to exposed healthy donors. As previously reported8 ML showed the highest reactivity with IgG1 antibodies (44-fold) followed by IgG3 (sixfold) antibodies in LL/BL patients compared to the control (HC) group. All recombinant antigens also evoked IgG1 responses in LL/BL patients compared to household contacts (HC). However, the greatest differential in the mean levels between the disease (LL/BL) and control group (HC) was observed with IgG1 antibodies to ML 10 K (32-fold higher). Interestingly, ML 65 K showed a strong IgG1 antibody response in LL/BL patients that was comparable to ML 10 K in terms of optical density units but this was only twofold higher than HC. Tuberculoid patients (BT/TT) did not show significantly raised IgG1 antibody subclass responses compared to HC with ML 10 K and ML 18 K using the Mann–Whitney test. BT/TT patients showed lower IgG1 responses to ML 65 K compared to HC, which did not achieve statistical significance. However, this trend could suggest a downregulation of IgG1 antibody responses in the BT/TT patient group to ML 65 K.
Table 1.
Comparison of IgG subclass responses to M. leprae recombinant antigens
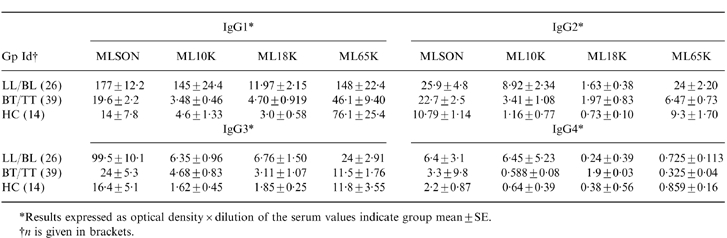
*Results expressed as optical density × dilution of the serum values indicate group mean±SE.
†n is given in brackets.
IgG2 antibodies were detected in LL/BL patients to ML10 K (≈eightfold) and ML 65 K (≈twofold) compared to HC. This is surprising as neither recombinant antigen is glycosylated and IgG2 responses are mainly directed to carbohydrate antigens.12 IgG2 antibody responses were not detected in LL/BL patients against ML 18 K.
An increase (two- to threefold) was observed for IgG3 antibodies for all three recombinant antigens in patients with lepromatous disease, suggesting that increases in IgG3 subclass antibody may be related to polyclonal activation of B-cell responses in lepromatous patients.8 As previously reported, the current study group also showed significant differences (Mann–Whitney test) in the polyclonal IgG1 (P < 0·0001) and IgG3 (P < 0·03) response in patients with lepromatous disease (data not shown) and would, thus, support this conclusion. The IgG4 response, which is a marker of Th2 activation, was generally low to all recombinant antigens with respect to optical density units. Patients with LL/BL disease showed a 10-fold higher optical density to ML 10 K but was not statistically significant. ML 18 K or ML 65 K showed a low level of activity, also suggesting that there is no generalized shift to Th2 antibody responses.
These results demonstrate that augmentation of IgG1 responses to the three HSPs tested is mainly restricted to patients with lepromatous disease. More interestingly, these results point towards inherent differences in the ability of different antigens to evoke IgG antibody subclasses. Among the three HSPs tested ML 10 K was a particularly strong inducer of IgG1 antibody subclass. We therefore carried out more detailed analysis of IgG1 antibody responses to ML 10 K.
Distribution of IgG subclass antibodies to ML 10 K
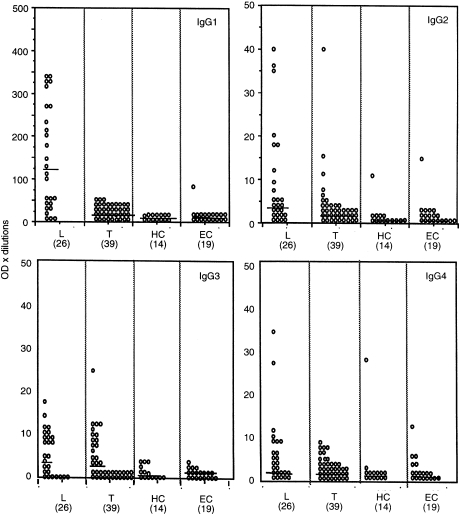
Figure 1 shows the IgG subclass antibodies to ML 10 K as a scatter plot and includes the responses of healthy endemic controls (EC) and healthy household contacts (HC) to analyse antibody responses associated with bacillus Calmette–Guèrin (BCG) vaccination, exposure to either M. leprae or other environmemtal mycobacteria. Several points emerged from these analysis. It was reassuring to observe that positive IgG subclass responses were observed in some donors to all four IgG subclass antibodies to ML 10 K, validating our detection system. Antibody responses were uniformly low in the EC group with all four IgG subclasses indicating low levels of cross-reactive antibodies (Fig. 1).
Figure 1.
IgG subclass antibody responses to Ml 10 K in leprosy patients with lepromatous and borderline lepromatous leprosy (L = 26), borderline tuberculoid and tuberculoid leprosy (T = 39) and healthy household contacts of leprosy patients (HC = 14) and healthy endemic controls (EC = 19). Results are shown as optical density×dilution of the serum. Each open circle represents an individual patient. Horizontal lines show median levels for each group.
It was also very clear that in addition to the magnitude of IgG1 response (Table 1) the frequency of positive antibody responses were also highest for IgG1 antibodies to ML 10 K. The cut-off value used to determine positive responses was mean+2SD of EC group. A majority (65%) of lepromatous patients showed >40 OD units. Very little response was observed in tuberculoid patients, suggesting that exaggerated IgG1 responses was restricted to patients with lepromatous disease.
IgG3 antibodies to ML 10 K were detected across the disease spectrum and showed similar concentrations in both lepromatous (50%) and tuberculoid (33%) patients. It was surprising to note that, in tuberculoid patients, no IgG1 response was detected, although a strong IgG3 response is observed.
Lepromatous patients (23%) also showed positive IgG4 antibody responses to ML 10 K. Thus, ML 10 K was able to induce positive antibody responses in all four IgG subclasses in leprosy patients. Both household contacts and endemic controls showed extremely low concentrations of antibodies for all four subclasses, indicating that augmentation of antibody responses are associated with progressive disease.
A small percentage of lepromatous patients (23%) also showed positive antibody responses in the IgG2 subclass. The presence of IgG2 antibodies to ML 10 K and 65 K was surprising because IgG2 antibodies are mainly directed to carbohydrate antigen14 and the recombinant antigens are not glycosylated. However, both the ML 65 K and the ML 10 K preparation did have considerable endotoxin (LPS) contamination (see Materials and Methods) which might bind IgG2 antibodies, while ML 18 K which was free of endotoxin contamination did not bind IgG2 antibodies. Thus, the specificity of binding of IgG2 to ML 10 K was investigated further.
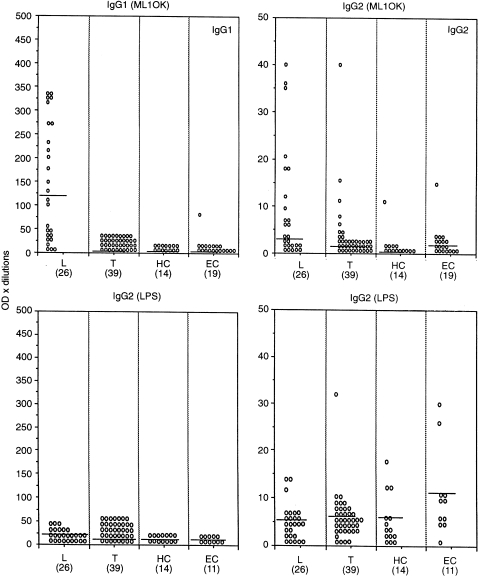
Figure 2 shows the results comparing IgG1 and IgG2 antibodies to ML 10 K and LPS in the same series of patients. It is quite clear that IgG1, which is the most significantly raised antibody subclass in LL/BL patients, does not show significant binding to LPS. More surprisingly, binding of IgG2 antibody to ML 10 K was also not related to LPS contamination. Very little IgG3 antibody was detected to LPS (data not shown), again indicating that IgG subclass antibodies were directed to the M. leprae antigens rather than to LPS contaminating the preparations.
Figure 2.
IgG1 and IgG2antibody subclass response to ML 10 K (top panels) and LPS (bottom panels). All other parameters are the same as in Fig. 1
ML 10 K epitopes recognized by IgG1 antibodies
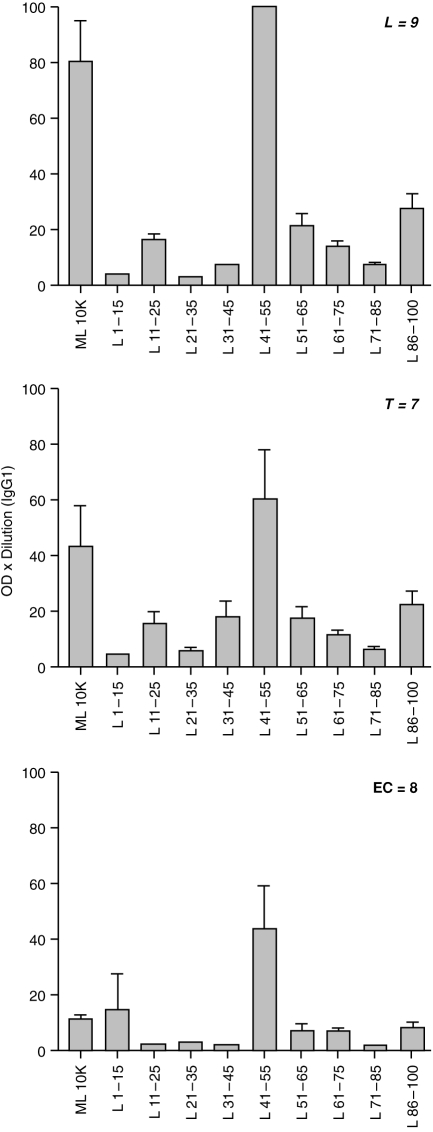
Because of the strong IgG1 response to ML 10 K we further analysed the epitopes being recognized by this particular IgG subclass. 15-mer overlapping synthetic peptides were used in the antibody analysis (Fig. 3). The strongest binding was detected with aa41–55 across the leprosy spectrum, with the highest response in patients with lepromatous disease. A low level of responses was also observed for two other peptides (aa86–100 and aa51–65). We were also able to detect a signal with IgG1 in healthy endemic controls, although we had previously observed minimal reactivity with the whole ML 10 K. This may be related to background noise which is relatively low with ML 10 K peptides.
Figure 3.
IgG1 antibody binding to ML 10 K and 15-mer peptide spanning the entire length of ML 10 K. Results are given as group mean±1 SEM and expressed as optical density×dilution of serum from patients with lepromatous and borderline lepromatous leprosy (L = 9), borderline tuberculoid and tuberculoid leprosy (T = 7) and healthy household contacts of leprosy patients (HC = 14) and healthy endemic controls (EC = 8).
Because ML 10 K shows >90% homology with M. tuberculosis 10 K (MT 10 K) we analysed IgG1 antibody responses in relation to the homology within the peptides.
IgG1 antibodies predominantly recognize shared ML and MT 10 K epitopes
Table 2 shows the amino acid sequence homology for the 15-mer overlapping peptides and the frequency of IgG1 antibody responses to both ML- and MT-derived peptides. The highest frequency of antibody responses was observed for common peptides in ML and MT 10 K (aa41–55 and aa 86–100) and positive responses were observed across the disease spectrum, as well as in the healthy endemic controls. The antibody response to aa51–65 which differed by three amino acids in ML, and MT 10 K was lower than the other peptides and was restricted to leprosy patients. However, it was interesting to observe that the antibody responses was marginally higher to the aa 51–65 MT homologue, again suggesting that the common epitopes, rather than ML-specific epitopes, are the targets of IgG1 antibody responses and that the differences in recognition across the disease spectrum as well as in healthy control group (EC) are restricted to the magnitude of response.
Table 2.
Recognition of M. leprae and M. tuberculosis 10 000 MW peptides by IgG1 antibodies
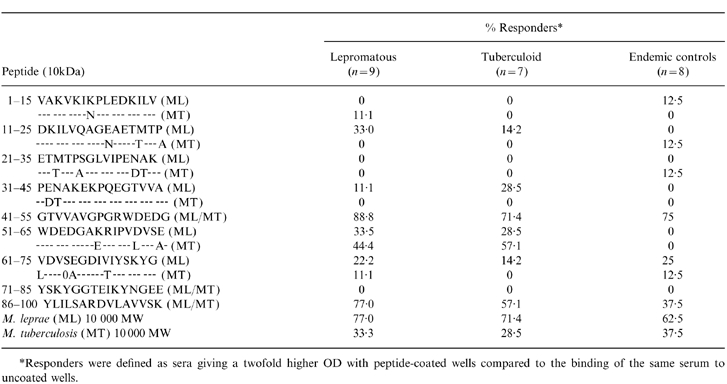
*Responders were defined as sera giving a twofold higher OD with peptide-coated wells compared to the binding of the same serum to uncoated wells.
DISCUSSION
Leprosy is caused by M. leprae which requires an intracellular habitat, the monocyte/macrophage system, to replicate within the host. As is the case with other intracellular infections,4,5 leprosy patients with disseminated disease show augmented antibody responses but T-cell anergy.2,15 The highest elevation in patients with lepromatous disease was observed for IgG1 antibodies against M. leprae sonicate, as previously described.8 Complement-fixing antibodies (IgG2a; a homologue of human IgG1) in the murine system have been shown to be regulated by interferon-′ (IFN-′), which is a Th1-secreted cytokine.16 It was therefore intriguing to observe augmentation of IgG1 antibodies (with complement-fixing ability), in patients with lepromatous disease in the absence of detectable Th1 responses. In this study we have, therefore, addressed the issue of the qualitative nature of IgG1 antibody responses to various recombinant purified antigens in leprosy across the disease spectrum as well as in healthy endemic controls. Our major findings were: (i) selective augmentation of IgG1 antibody responses to ML10 K; (ii) recognition of a restricted number of epitopes across the disease spectrum and healthy controls by IgG1 antibodies; (iii) dominant recognition of cross-reactive epitopes, which were common to both ML and MT 10 K.
We have looked at two control groups: (i) healthy household contacts of patients with active disease to distinguish antibodies evoked by exposure versus active disease; and(ii) healthy endemic controls to establish baseline antibody levels from exposure to cross-reactive environmental mycobacteria and BCG vaccination. T-cell responses have been previously shown to be extremely high in both control groups.17 Although baseline levels were relatively high for all four IgG subclasses when M. leprae sonicate or ML 65 K was used as antigen, ML 10 K and ML 18 K, on the other hand, showed very low baseline antibody responses in HC. This would suggest that exposure to M. leprae was sufficient to induce detectable antibody responses to ML 65 K antigen while considerable bacillary load was required for detectable responses to ML 10 K and ML 18K. This seemed to be particularly true for ML 10 K. What was surprising was the augmentation of IgG1 response to ML 10 K only in patients with lepromatous disease, although IgG3 antibody responses were detected in both lepromatous and tuberculoid leprosy patients. Because IgG3 antibody responses are switched to IgG1 antibodies it would seem that in lepromatous patients this switching is occurring, while in tuberculoid patients this event may not take place. Switching of IgG3 to IgG1 in humans has been shown to be dependent on IL-10,18 a cytokine secreted by both macrophages and the Th2 subset. This cytokine inhibits macrophage function by downregulating major histocompatibility complex (MHC) class II presentation19 and inhibits secretion of cytokines by the Th1 subset.20 IL-10 has also been shown to activate B cells at all stages of maturation.21 We have observed similar elevation of IL-10 secretion in ML 10 K-stimulated peripheral blood mononuclear cells (PBMCs) (manuscript in preparation), in both groups of leprosy patients (lepromatous and tuberculoid), but very little IL-5 secretion, which is a marker for Th2 activation.22 One explanation, therefore, for IgG1 antibody responses in patients with lepromatous disease could be secretion of IL-10 by M. leprae infected macrophages, which may result in switching of IgG3 to IgG1. However, if this were occurring we should have observed a greater diversity in IgG1 antibody response. In fact, our results clearly demonstrate that the antibody responses were highly restricted to a few epitopes which were shared epitopes in ML and MT 10 K. This observation favours expansion of postswitched B cells rather than switching of IgG3 to B cells to IgG1-secreting B cells. We have also observed IL-6 which is both a proliferation and differentiating factor to be secreted in response to ML 10 K in PBMC-stimulated cultures. The highest elevation was observed in patients with lepromatous disease (manuscript in preparation). Thus, co-ordinate expression of IL-10 and IL-6 secreted by macrophages, rather than T-cell cytokines, may be responsible for the augmented IgG1 responses to ML 10 K. This also seems a more likely explanation, in light of the fact that infected macrophages with high bacterial loads have poor antigen-presenting capabilities, as would be the case in patients with lepromatous disease. As yet it is unclear if IgG1 antibodies are surrogate markers of disseminated disease or if they play a direct role in disease progression. Macrophages have high affinity receptors for IgG1 and IgG3 antibodies and may facilitate uptake of opsonized bacteria and subsequent activation and expression of cytokines. ML 10 K, therefore, provides a useful model to study the regulation of IgG1 antibody responses in leprosy and may provide important insights into the mechanism of disease pathogenesis. Detailed correlation analysis of cytokine expression by both macrophage (IL-12, tumour necrosis factor-α (TNF-α), IL-6 and IL-10), as well as T-cell cytokines (IFN-γ, IL-2, I-L5) induced by ML 10 K and IgG1 antibody responses to ML 10 K are in progress.
The authors would like to thank The Marie Adelaide Leprosy Centre, Karachi, Pakistan for patient material, Dr Sebastian Lucas, University College School of Medicine, London, UK (currently St Thomas's Hospital) for providing histopathology on all leprosy patients, Mr Philip Broadbent for ranking the peptides, Syed Asif Hussain for data analysis and graphics, Miss Regina Paul for secretarial help. Excellent technical assistance was provided by Miss Nabila Abrar and Miss Maqboola Dojki. Useful suggestions on the manuscripts by Ms Arnawaz Kaleem and Dr Sarwat Jamil are gratefully acknowledged.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
The authors would like to thank The Marie Adelaide Leprosy Centre, Karachi, Pakistan for patient material, Dr Sebastian Lucas, University College School of Medicine, London, UK (currently St Thomas's Hospital) for providing histopathology on all leprosy patients. Mr Philip Broadbent for ranking the peptides, Syed Asif Hussain for data analysis and graphics, Miss Regina Paul for secretarial help. Excellent technical assistance was provided by Miss Nabila Abrar and Miss Maqboola Dojki. Useful suggestions on the manuscripts by Ms Arnawaz Kaleem and Dr Sarwat Jamil are gratefully acknowledged.
REFERENCES
- 1.Bullock WE, Jr, Ho MF, Chin MJ. Studies of immune mechanisms in leprosy. II Quantitative relationship of IgG, IgM and IgA immunoglobulins. J Lab Clin Med. 1970;75:863. [PubMed] [Google Scholar]
- 2.Melsom R, Harboe M, Myrvang B, Godal T, Belehu A. Immunoglobulin class specific antibodies to M. leprae in leprosy patients, including the indeterminate groups and healthy contacts as a step in development of methods for serodiagnosis of leprosy. Clin Exp Immunol. 1982;47:225. [PMC free article] [PubMed] [Google Scholar]
- 3.Bullock WE, Watson S, Nelson KE, Schauf V, Makonkawkeyoon S, Jacobson RR. Aberrant immunoregulatory control of B lymphocyte function in lepromatous leprosy. Clin Exp Immunol. 1982;49:105. [PMC free article] [PubMed] [Google Scholar]
- 4.Heinzal FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. J Exp Med. 1989;169:59. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romani L, Menacacci A, Cenci E, Spaccapelo R, Mosci P, Bistoni F. CD4+ subset expression in murine candidiasis. J Immunol. 1993;150:925. [PubMed] [Google Scholar]
- 6.Snapper CM, Finkleman FD. Immunoglobulin class switching. In: Paul WE, editor. Fundamental Immunology. 3. New York: Raven Press Ltd; 1993. p. 855. [Google Scholar]
- 7.King CL, Nutman TB. IgE and IgG subclass regulation by IL4 and IFN-′ in human helminth infections. J Immunol. 1993;151:458. [PubMed] [Google Scholar]
- 8.Hussain R, Kifayet A, Chaing TJ. IgG1 and IgG3 are the markers of progressive disease in leprosy. Infect Immun. 1995;63:41. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates ARM. Immunological aspects of Chaperonins. In: Ellis RJ, editor. The Chaperonins. London, UK: Academic Press Inc; 1996. [Google Scholar]
- 10.Young D, Lathigra R, Hendrix R, Sweetser D, Young RA. Stress protein are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci USA. 1988;85:4267. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain R, Jamil S, Kifayet A, et al. Quantitation of IgM antibodies to the M. leprae synthetic dissacharide can predict early bacterial multiplication in leprosy. Int J Lep. 1990;58:49. [PubMed] [Google Scholar]
- 12.Hussain R, Poindexter RW, Wistar R, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. I Development of two-site immunoenzymometric assay for total IgG subclass determinants. J Immunol Methods. 1986;93:8. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- 13.Hussain R, Poindexter RW, Ottesen EA, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. Enzyme immunoassay to define antigen specific (anti-filarial) IgGsubclass antibodies. J Immunol Methods. 1986;94:73. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- 14.Barrett DJ, Ayoub EM. IgG2 subclass restriction of antibody to pneumoccal polysaccharides. Clin Exp Immunol. 1973;63:127. [PMC free article] [PubMed] [Google Scholar]
- 15.Myrvang B, Godal T, Ridley DS, Froland SS, Song YK. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973;14:541. [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-′ regulates the isotypes of Ig secreted in vivo humoral immune responses. J Immunol. 1988;140:1022. [PubMed] [Google Scholar]
- 17.Hussain R, Dockrell HM, Shahid F, Zafar S, Chiang TJ. Leprosy patients with lepromatous disease recognise cross reactive T cell epitopes in the M. leprae 10kDa antigen. Clin Exp Immunol. 1998;114:708. doi: 10.1046/j.1365-2249.1998.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briere F, Servet-Derprat C, Bridon JM, Saint Remy JM, Bunchereau J. Human IL10: induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Waal-Malefyt R, Haanen J, Spits H, et al. IL10 and viral IL10 strongly reduce antigen specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down regulation of class II MHC expression. J Exp Med. 1991;174:915. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore JW, De Waal-Malefyt R, Vieira P, Mosmann TR. Interleukin 10. Ann Rev Immunol. 1993;11:165. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 21.Burdin N, Van Kooten C, Galibert L, et al. Endogenous IL6 and IL10 contribute to the differentiation of CD-40 activated human B lymphocytes. J Immunol. 1995;154:2533. [PubMed] [Google Scholar]
- 22.Dockrell HM, Young SK, Britton K, et al. Induction of Th1 cytokine responses by mycobacterial antigens in leprosy. Infect Immun. 1996;64:4385. doi: 10.1128/iai.64.10.4385-4389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]