Abstract
Interleukin-6 (IL-6), a multifunctional cytokine, is induced in the acute-phase reaction following ultraviolet (UV) irradiation of humans and mice. Using IL-6-deficient (IL-6−/−) mice, we investigated the role of IL-6 in immunosuppression and inflammatory responses caused by UVB (280–320 nm) radiation. The IL-6−/− mice had a defective contact hypersensitivity (CHS) in response to the sensitizers 2,4-dinitrofluorobenzene and oxazolone. The injection of recombinant IL-6 (rIL-6) into these mice resulted in a marked recovery of the CHS. Serum IL-6 was significantly elevated by UV irradiation of wild-type B6 J/129Sv (IL-6+/+) mice but was not detectable in IL-6−/− mice. Interestingly, there was no induction of serum interleukin-10 (IL-10) by UV irradiation of IL-6−/− mice, whereas UV exposure caused a significant increase in serum IL-10 levels in IL-6+/+ mice. Injection of rIL-6 into IL-6−/− mice increased IL-10 to levels similar to those of IL-6+/+ mice. Being different from IL-6+/+ mice, no epidermal proliferation was found at 48 hr in the IL-6−/− mice, but delayed cell proliferation was observed at 72 hr after UV exposure. Immunohistochemical analysis demonstrated that the epidermis was capable of synthesizing IL-6 at 72 hr after UV irradiation of IL-6+/+ mice. In addition, the IL-6-positive cells appeared to be Langerhans' cells, which were detected with dendritic cell-reactive S-100 antibody. The present study strongly suggests that IL-6 may play a crucial role in the alteration of cutaneous immune responses following UV exposure, and provides evidence that IL-6 is a potent inducer of IL-10. Furthermore, IL-6 production induced by UV radiation appears to be an important early signal for repair of UV-caused skin damage.
INTRODUCTION
Exposure of the skin to ultraviolet (UV) radiation results in the induction of inflammation and immunosuppression. These responses are known to be mediated via the induction of cytokines,1,2 and are exemplified by an impairment of the ability to react to contact allergens.3 Normally, cutaneous cytokines activate epidermal Langerhans' cells (LC), which function as antigen-presenting cells (APC) and then generate T-cell-mediated immune reactions such as contact hypersensitivity (CHS). However, impairment of the CHS reaction by UV exposure has been well documented.4–6 The UVB-induced inhibitory effect on APC function of LC is considered to underlie the immune suppression.5 After exposure to UV radiation, epidermal cells appear to release a variety of cytokines, such as interleukin (IL)-1β, tumour necrosis factor-α (TNF-α) and IL-10, which may result in suppression of the immune responses.3,7,8 IL-6, a cytokine with multiple effects, is one of the cytokines most readily inducible in a wide number of cell types, and has been reported to be involved in immune responses.9 IL-6 is also known to be induced in cutaneous inflammatory diseases like psoriasis10 and to play a crucial role in the normal CHS reaction in oxazolone-treated mice.11
It is reported that some cytokines, primarily IL-1β, TNF-α IL-6 and IL-10, participate in immune and inflammatory responses. IL-1β,12 IL-69 and TNF-α13 are important proinflammatory cytokines. It has been reported that production of IL-1β and IL-6 was induced in humans following UV irradiation.14,15 IL-1β is reported to be a potent inducer of IL-6 and to have synergistic effects with IL-6.9 There is much evidence that IL-10 participates in immune suppression following UV exposure.7,8 The phenomenon of systemic suppression of immune functions by UV radiation is well established in animals and humans, but the mechanism(s) by which this immunological impairment is produced remain undefined.
IL-6−/− mice generated by gene targeting16 are available and should provide significant information on the relationship between cytokines IL-1, IL-6 and IL-10 in inflammatory and immune responses induced by UV radiation. The aim of this study was therefore to determine the mutual roles of IL-6 in the CHS response, and in UV-induced immune suppression.
MATERIALS AND METHODS
Mice
IL-6-deficient mice (IL-6−/−) and B6J/129Sv mice (IL-6+/+), as controls, were purchased from the Jackson Laboratories (Bar Harbor, ME) and bred in the National Institute for Environmental Studies. The IL-6−/− mice were viable, fertile and phenotypically normal. Mice were maintained under standard conditions of a 12-hr light–dark cycle, temperature at 23±1° and humidity at 55±10%. Female mice aged 8 weeks old were used, and at 24 hr before irradiation or sensitization, fur was removed from the dorsum or abdomen, respectively, with clippers. The mice received human care throughout the experiment according to the guidelines of the National Institute of Environmental Studies (Tsukuba, Japan).
UV radiation
UV radiation was provided by unfiltered fluorescent UVB tubes (F15TA.UV-B, 280-320 nm, SANKYO, Japan). Irradiation was measured at 310 nm using a UVX radiometer (UV Products Inc., San Gabriel, CA). Mice received one daily minimal oedematous dose (MED) of UVB radiation, and the cumulative dose for three consecutive days was 7·2 kJ/m2. The MED had been previously determined from a series of increasing UVB exposures by measuring the mid-dorsal skinfold thickness of IL-6+/+ mice at 24 hr postirradiation, using a spring micrometer (Mitutoyo Co., Kawasaki, Japan). No excessive heat was produced during irradiation.
Recombinant mouse IL-6 (rIL-6; a kind gift from Central Research Laboratories of Ajinomoto Co., Yokohama, Japan), of specific activity 5 U/ng, was freshly dissolved in phosphate-buffered saline (PBS: 10 mm potassium phosphate buffer, pH 7·3, 150 mm NaCl) containing 2% mouse serum.17 IL-6−/− mice were injected intraperitoneally (i.p.) with rIL-6 (5 μg per mouse in a total volume of 100 μl), 20 min before sensitization, for 2 days and once before challenge. Control mice received the same volume of 2% mouse serum in PBS. To investigate IL-10 induction by UV irradiation of IL-6−/− mice, four IL-6−/− mice were injected with rIL-6 (5 μg/mouse) prior to UV exposure.
Induction of contact hypersensitivity
Contact hypersensitivity was determined by the method previously described.18 Briefly, five or six mice from each group of 10 or 12 (the remaining animals acting as nonirradiated controls) received one MED of UVB radiation, daily, on days 1–3. On days 8 and 9, mice were sensitized with either of two contact sensitizers: 50 μl of 0·15% (v/v) 2,4-dinitrofluorobenzene (DNFB; Sigma, St Louis, MO) in acetone, or 100 μl of 1% oxazolone (Sigma) in ethanol. Sensitization was on the largest possible area of the shaved, non-irradiated abdominal skin. The mice were challenged on day 15 with 5 μl of freshly prepared sensitizer, which was applied to each surface of both pinnae. Both the prechallenge ear thickness and the maximum ear thickness at 19–20 hr were measured using a spring micrometer. The mean value±SD for differences in the ear thickness (average ear swelling) was calculated.
Serum IL-6, IL-1β and IL-10 measurement
Serum was collected from three or four mice from each group at various time-points after UV exposure (7·2 kJ/m2), and the concentrations of IL-6, IL-1β and IL-10 were determined using respective BIOTRAK™ enzyme-linked immunosorbent assay (ELISA) kits (Amersham Pharmacia Biotech, Bucks, UK), according to the manufacturer's instructions.
Immunohistochemical staining
Dorsal skin samples of three mice from each group were collected at 12, 24, 48 and 72 hr after UV irradiation (7·2 kJ/m2), fixed in HistoChoice (Amresco, Parkway, OH) and embedded in paraffin. Deparaffinized 5-mm tissue sections were subjected to immunohistochemical staining with anti-IL-6 mouse immunoglobulin G (IgG) (I-3393, Sigma) and anti-S-100 (S-2644, Sigma) for IL-6 and dendritic cells, respectively. The bound primary antibodies were then visualized with the avidin-biotin peroxidase complex (ABC) immunostaining method (PK-4000, Vector Lab., Burlingame, CA) as described previously.19 The number of cell layers in the epidermis was counted from five fields of each slide stained with haematoxylin and eosin (H&E).
Statistical analysis
Comparisons of mean values were performed by Student’s t-test for independent samples.
RESULTS
Impaired CHS in IL-6−/− mice and restoration of CHS by injection of rIL-6
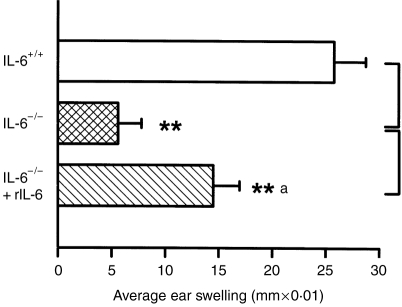
The contribution of IL-6 to the normal CHS response, and to suppression of the CHS following UV irradiation, was assessed by the comparison of IL-6+/+ and IL-6−/− mice. The normal CHS to DNFB was clearly impaired in IL-6−/− mice (Fig. 1), the average ear swelling response being reduced to 31% of that of the IL-6+/+ mice. Exposure to UV radiation suppressed the CHS by 47% in IL-6+/+ mice, but the suppression of the already defective ear swelling in IL-6−/− mice after UV irradiation appeared not to be significant. A similar finding was obtained with another sensitizer, oxazolone (Fig. 2), suggesting an essential role of IL-6 in the induction of the CHS response. To further evaluate the role of IL-6 in the CHS, we injected rIL-6 into IL-6−/− mice and observed that the injected rIL-6 dose, partly, but significantly, restored the induction of the CHS (Fig. 2).
Figure 1.
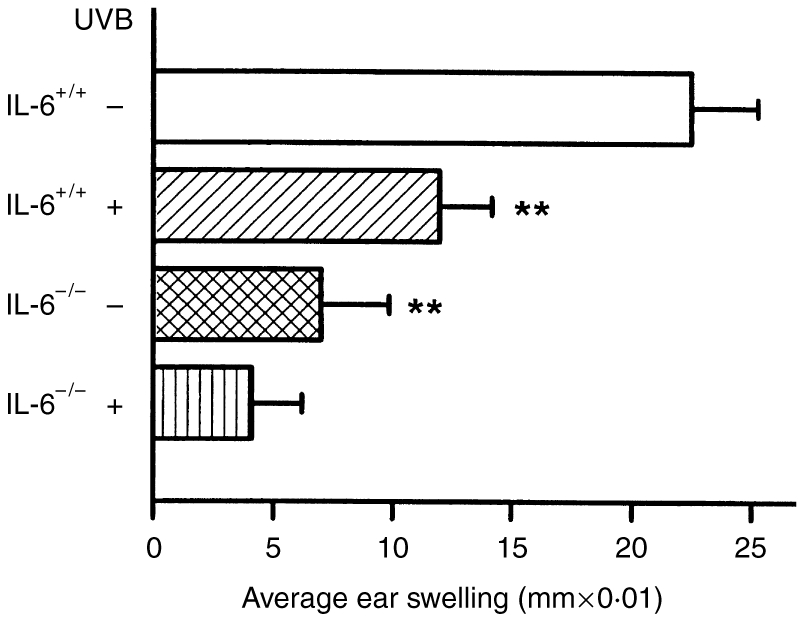
Reduced contact hypersensitivity (CHS) to 2,4-dinitrofluorobenzene (DNFB) in interleukin (IL)-6−/− mice with and without exposure to UV radiation. A severely impaired CHS reaction was found in the IL-6−/− mice compared with IL-6+/+ mice. UV exposure suppressed the CHS response in IL-6+/+ mice. The data represent mean±SD for five mice. **Indicates a significant difference (P <0·01) from non-UV-irradiated IL-6+/+ mice.
Figure 2.
Restoration of contact hypersensitivity (CHS) to oxazolone in recombinant interleukin-6 (rIL-6)-injected mice. IL-6−/− mice injected with rIL-6 showed significant restoration of the CHS response to oxazolone. The data represent mean±SD for six mice.** and **a indicate a significant difference (P < 0·01) from IL-6+/+ and IL-6−/− mice, respectively.
Changes in serum levels of IL-6 and IL-1β in IL-6+/+ and IL-6−/− mice following UV irradiation
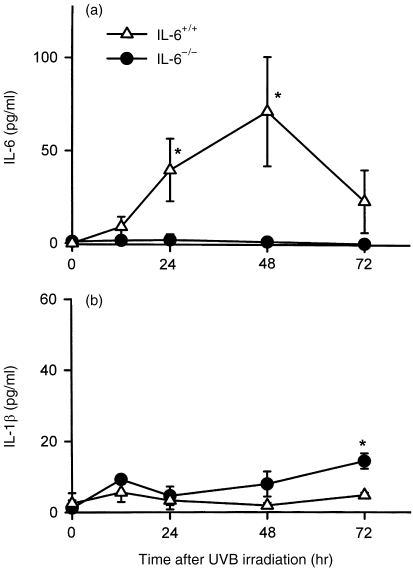
Because IL-1β and IL-6, are known to be the most important proinflammatory cytokines, we measured serum IL-6 and IL-1β levels at various time-points after UV irradiation. In IL-6+/+ mice, UV irradiation caused a marked increase in the level of serum IL-6, which peaked at 48 hr and returned to almost basal levels by 72 hr (Fig. 3a). As expected, IL-6 was not detectable in the serum of IL-6−/− mice, even after UV irradiation. UV irradiation of IL-6−/− mice also tended to increase serum IL-1β levels, until at least 72 hr after irradiation, but did not cause any significant change in IL-6+/+ mice (Fig. 3b).
Figure 3.
Changes in serum interleukin (IL)-6 and IL-1β levels in IL-6−/− and IL-6+/+ mice at various time-points following UV exposure. (a) Serum IL-6 was elevated by UVB irradiation and peaked at 48 hr in IL-6+/+ mice, but was not detectable in IL-6−/− mice. (b) Serum IL-1β increased slightly following UV exposure in IL-6−/− mice in comparison with IL-6+/+ mice. The data represent mean±SD for three mice. *Indicates a significant difference (P < 0·05) from IL-6+/+ or IL-6−/− mice.
Changes in serum IL-10 levels of IL-6+/+ and IL-6−/− mice following UV irradiation
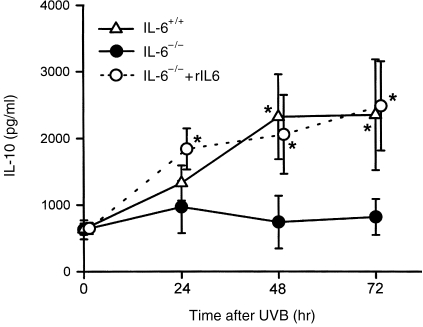
As IL-10 production was reported to be closely correlated with suppression of immune function by UV irradiation,8 changes in serum IL-10 levels were measured following UV exposure. Interestingly, as shown in Fig. 4, there was no increase of IL-10 in the serum of IL-6−/− mice at any time following UV irradiation, whereas IL-10 levels of IL-6+/+ mice were significantly elevated at 48 hr and 72 hr post-UV. These unexpected results suggested that IL-6 might play a role in the UVB upregulation of IL-10, and to confirm this notion we injected IL-6−/− mice with rIL-6 and evaluated the concentration of serum IL-10. It was found that injection of rIL-6 resulted in the production of normal postirradiation levels of IL-10 in IL-6−/− mice.
Figure 4.
Induction of serum interleukin (IL)-10 in IL-6−/− mice injected with recombinant interleukin-6 (rIL-6) at various time-points following UV exposure. There was no induction of serum IL-10 in IL-6−/− mice after UV exposure, although IL-10 was dramatically induced by UV irradiation of IL-6+/+ mice. Injection of rIL-6 significantly induced serum IL-10 levels at 24, 48 and 72 hr following UV exposure in IL-6−/− mice. The data represent mean±SD for four mice. *Indicates a significant difference (P <0·05) from IL-6−/− mice.
Histopathology of the UV-induced inflammatory response in IL-6+/+ and IL-6−/− mouse skin
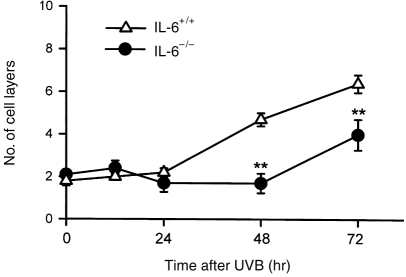
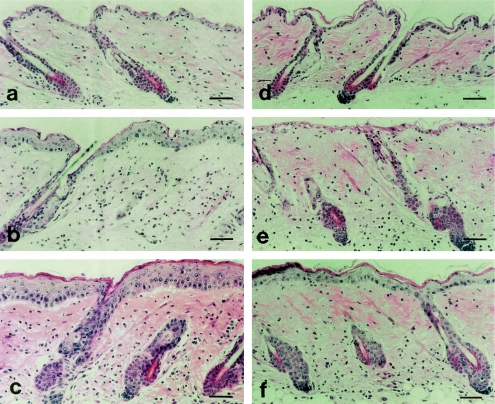
UV irradiation caused epidermal hyperplasia and infiltration of inflammatory cells, fibroblasts, neutrophils and macrophages in the dermis of IL-6+/+ mice. At 48 hr after UV irradiation, a large number of mitotic cells was found in the basal layer, and an increased number of cell layers was evident in the epidermis (Figs 5 and 6b), the thickness of which had increased by 72 hr (Fig. 6c). By contrast, in IL-6−/− mice, both macroscopic and microscopic examinations revealed severe skin damage as a result of UV irradiation. No epidermal proliferation was found up to 48 hr post-UV in the IL-6−/− mice, and inflammatory cells were found in the dermis (Fig. 6e). Interestingly, we observed the appearance of mitotic cells in the epidermal basal layer at 72 hr after UV irradiation of IL-6−/− mice, and a delayed increase in the number of cell layers was found (Figs 5 and 6f). These results confirmed the direct contribution of IL-6 to epidermal hyperplasia and the repair of UV-damaged skin. IL-6 production induced by UV exposure may be an important signal that co-ordinates activities of macrophages and lymphocytes to repair UV-damaged skin at an early stage.
Figure 5.
Defective epidermal proliferation in interleukin (IL)-6−/− mice following UV exposure. Unlike IL-6+/+ mice, IL-6−/− mice had no epidermal hyperplasia until 48 hr post-UVB, followed by a delayed increase at 72 hr post-UVB. The data represent mean±SD for three mice. **Indicates a significant difference (P <0·01) from IL-6+/+ mice.
Figure 6.
Greater skin damage by UV exposure in interleukin (IL)-6−/− mice compared with IL-6+/+ mice. (a–c) Skin of IL-6+/+ mice at 24, 48 and 72 hr after UV exposure, respectively. (d–f) Skin of IL-6−/− mice at 24, 48 and 72 hr after UVB exposure, respectively. Haematoxylin and eosin (H&E) staining showed the absence of epidermal proliferation at 48 hr following UV exposure and the appearance of delayed epidermal proliferation at 72 hr in the IL-6−/− mice. IL-6−/− mice had greater severity of skin damage on gross appearance than IL-6+/+ mice. Bar, 50 μm.
IL-6 induction in dendritic cells by UV irradiation
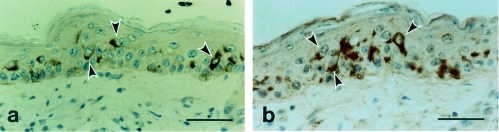
As UV irradiation elevated the serum concentration of IL-6 in IL-6+/+ mice, we sought to identify, immunohistochemically, the cells that synthesize and release this cytokine in the skin. Immunohistochemical staining was negative in untreated skin. Interestingly, immunostaining was found, 72 hr after UV exposure, in IL-6+/+ mice but not in IL-6−/− mice, indicating induction of IL-6 in the cytoplasm of epidermal cells, but not in the dermis (Fig. 7a). Furthermore, S-100 antibody-reactive cells, which bind specifically to dendritic cells, probably either LC or melanocytes, appeared to be localized in the epidermis, similarly to the IL-6-positive cells (Fig. 7b).
Figure 7.
Positive immunostaining for interleukin (IL)-6 and dendritic cells at 72 hr following UV exposure in IL-6+/+ mice. (a) Immunostaining showed the induction of IL-6 at 72 hr after UV irradiation. Arrowheads indicate the IL-6 positive cells. (b) Immunostaining with S-100 antibody confirmed that IL-6 was induced by UV irradiation in epidermal dendritic cells, probably Langerhans' cells (arrowheads). Bar, 50 μm.
DISCUSSION
A variety of cytokines, in particular IL-1β,12 IL-620 and IL-10,3,21,22 have been described to act as mediators for immune reactions of the skin. In the present study we investigated possible involvement of these cytokines in the suppression of immunity caused by UV radiation, by utilizing the systemic CHS response as a typical hallmark. Initially, we observed that the CHS reaction in the IL-6−/− mice was profoundly suppressed, suggesting a major role of IL-6 in the induction and/or elicitation of this response. Restoration of the CHS response by injection of rIL-6 provided additional evidence of the role of IL-6 in CHS, which is consistent with the results of Holliday et al.11 that demonstrated a time- and dose-dependent increase in cutaneous IL-6 following topical oxazolone treatment.
Following UV irradiation, we observed not only a strong suppression of the CHS reaction, but also a marked increase of serum IL-6 in IL-6+/+ mice, peaking at 48 hr and returning to near-baseline levels by 72 hr. As IL-1β is reported to be a potent inducer of IL-6 in fibroblasts23 and keratinocytes,20 the involvement of IL-1β in this production of serum IL-6 was investigated. UVB irradiation caused a relatively modest increase in levels of serum IL-1β at 12 hr in IL-6+/+ mice but no correlation with time was found with the appearance of serum IL-6, indicating no more than a minor participation of IL-1β in the suppression of CHS. In fact, the moderate appearance of IL-1β post-UV irradiation, in the IL-6−/− mouse was significantly enhanced at 72 hr compared with the IL-6+/+ mouse, further suggesting a lack of dependence between IL-1β and IL-6, and perhaps some redundancy of these two cytokines, observable in the absence of IL-6.
In contrast, Enk et al.12 have shown that intradermal injection of IL-1β resulted in the same cytokine patterns released in mouse skin as found following treatment with the contact allergen trinitrochlorobenzene (TNCB). Antibody to IL-1β completely prevented TNCB sensitization in the mice, indicating an essential role for IL-1β in this primary immune response. There is thus a discrepancy between the results of Enk et al. and our study, perhaps owing to the timing of the measurements of cytokine responses: for example, an increase in the level of IL-1β mRNA was detected as early as 15 min after allergen application,12 and serum IL-1β concentration was found to be increased in response to UVB irradiation, peaking at 1–4 hr and returning to baseline levels by 8 h.24 The earliest measurement at 12 hr in the present study might not have identified an earlier increase, and therefore there remains the possibility of involvement of IL-1β with the CHS reaction.
There is substantial evidence to show that IL-10 is the key cytokine responsible for UV radiation-induced immune suppression.3,5,7,8,25 Ullrich3 has summarized the role of keratinocyte-derived cytokines in initiating the immune and inflammatory reactions caused by UVB irradiation, and suggests that cytokines originating in the epidermis can block the ability of LC to present antigen to T helper-1 lymphocytes, whereas the T helper-2-derived cytokines, such as IL-4 and IL-10, act to inhibit the induction of inflammatory immune reactions.1,25 UV irradiation induced both IL-10 mRNA and protein in cultured keratinocytes, and anti-IL-10 protected mice from the suppressive effect of the culture supernatant on delayed type hypersensitivity responses in mice,26,27 pinpointing IL-10 as a critical cytokine in UV-induced immune suppression.7 We therefore sought evidence for a possible relationship between IL-6 and IL-10 in the UV induction of immune suppression, and consequently detected, in IL-6+/+ mice, a dramatic increase in serum IL-10 by 24 hr post-UV irradiation, with further increase at least until 72 hr. Although there was no change in the serum IL-10 concentration of IL-6−/− mice after UV irradiation, serum IL-10 production was restored to the level of the IL-6+/+ mice by injection of rIL-6 into the IL-6−/− mice. It is suggested that IL-6 plays a role in immune suppression, after UV irradiation, by its essential requirement in UV-induced IL-10 production, which might be achieved by its action on the T helper-2 cell population, IL-6 being an important co-stimulator of lymphocyte activation.28,29 However, it was not possible to demonstrate IL-6 dependence of the suppression of CHS by UV in the already subnormal response of IL-6−/− mice.
Immunohistochemical staining for IL-6 was negative in untreated skin of IL-6+/+ mice, but localized to the cells in the epidermis at 72 hr post-UV exposure, with diffuse staining in the cytoplasm. The immunoreactive cells appeared to be dendritic, and to have a localization similar to cells that stained positively with the S-100 antibody, suggesting that they are LC (not melanocytes), consistent with the immunologically relevant changes in the mice. Keratinocytes have been reported as a major source of IL-6,20,30 but IL-6 has also been found in monocytes/macrophages, fibroblasts, dermal endothelial cells, sweat duct cells, and in all skin cell layers.9,31 In psoriasis, IL-6 was also found in endothelial cells, dermal infiltrate and keratinocytes,10 but IL-6 mRNA was detected in the LC isolated from murine epidermal cell culture,32 and inducible expression of IL-6 was observed in LC and lymph node dendritic cells.33 The present study has suggested that IL-6 is induced in the LC after UV exposure. The variations in immunohistological localization found by other investigators may reflect differences in induction stimuli and tissues examined.
We found that UV irradiation gave a rise in a significantly reduced hyperplasia, which persisted for at least 72 hr and was accompanied by greater severity of skin damage in the IL-6−/− mice in contrast to a rapid and strong hyperplastic response in UV-irradiated IL-6+/+ mice. A direct contribution of IL-6 to keratinocyte proliferation was described in psoriatic skin34 and cultured keratinocytes.10 The cultured keratinocytes were shown to proliferate seven times more rapidly in response to added IL-6, and thus IL-6 was thought to have a direct link to psoriatic lesional hyperplasia. The present findings also support the notion that IL-6 has a primary contribution to epidermal hyperplasia and the repair of UV-damaged skin of mice.9
In conclusion, this study shows that IL-6 plays an essential role in normal, cutaneous immune responses, appears to be a necessary factor for the production of IL-10 induced by UV radiation, and is important in the cell proliferative requirement for repair of cutaneous UV-induced damage.
Acknowledgments
This work was supported in part by the Science and Technology Agency in Japan and the University of Sydney Cancer Research Fund in Australia.
Abbreviations
- APC
antigen-presenting cell
- CHS
contact hypersensitivity
- DNFB
2,4-dinitrofluorobenzene
- H&E
haematoxylin and eosin
- IL-6
interleukin-6
- rIL-6
recombinant mouse interleukin-6
- IL-6−/−
interleukin-6-deficient mouse
- IL-6+/+
wild-type mouse
- LC
Langerhans' cell
- UV
ultraviolet.
REFERENCES
- 1.Luger TA, Schwarz T. Effect of UV light on cytokines and neuroendocrine hormones. In: Krutmann J, Elmets C, editors. Photoimmunology. Oxford: Blackwell; 1995. p. 55. [Google Scholar]
- 2.Noonan FP, Defabo EC. UV-induced immunosuppression. Relationships between changes in solar UV spectra and immunologic responses. In: Young AR, Bjorn LO, Moan J, Nultsh W, editors. Environmental UV Photobiology. New York, NY: Plenum Press; 1993. p. 113. [Google Scholar]
- 3.Ullrich SE. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultraviolet radiation-induced immune suppression. Photochem Photobiol. 1995;62:389. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher MS, Kripke ML. Systemic alteration induced in mice by UV light irradiation and its relationship to UV carcinogenesis. Proc Natl Acad Sci USA. 1977;74:1688. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black HS, degruijl FR, Forbes PD, et al. New trends in photobiology (invited review) Photocarcinogenesis: an overview. Photochem Photobiol. 1997;40:29. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 6.Noonan FP, Defabo EC, Kripke ML. Suppression of contact hypersensitivity by UV radiation and its relationship to UV-induced suppression of tumor immunity. Photochem Photobiol. 1981;34:683. [PubMed] [Google Scholar]
- 7.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865. [PubMed] [Google Scholar]
- 8.Rivas JM, Ullrich SE. The role of IL-4, IL-10 and TNF-α in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. 1994;56:769. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 9.Paquet P, Pierard GE. Interleukin-6 and the skin. Int Arch Allergy Immunol. 1996;109:308. doi: 10.1159/000237257. [DOI] [PubMed] [Google Scholar]
- 10.Grossman RM, Krueger J, Yourish D, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci USA. 1989;86:6367. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holliday MR, Dearman RJ, Basketter DA, Kimber I. Stimulation by oxazolone of increased IL-6, but not IL-10, in the skin of mice. Toxicology. 1996;106:237. doi: 10.1016/0300-483x(95)03191-h. [DOI] [PubMed] [Google Scholar]
- 12.Enk AH, Angeloni VL, Udey MC, Katz SI. An essential role for Langerhans' cell-derived IL-1β in the initiation of primary immune responses in skin. J Immunol. 1993;150:3698. [PubMed] [Google Scholar]
- 13.Rothe J, Lesslauer W, Lotscher H, et al. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 14.Urbanski A, Schwarz T, Neuner P, Krutmann J, Kirnbauer R, Kock A. Ultraviolet light induced increased circulating interleukin-6 in humans. J Invest Dermatol. 1990;94:808. doi: 10.1111/1523-1747.ep12874666. [DOI] [PubMed] [Google Scholar]
- 15.Ansel JC, Luger TA, Green I. The effect of in vitro and in vivo UV irradiation on the production of ETAF activity by human and murine keratinocytes. J Invest Dermatol. 1983;81:519. doi: 10.1111/1523-1747.ep12522862. [DOI] [PubMed] [Google Scholar]
- 16.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 17.Yasueda H, Nagase K, Hosoda A, Akiyama Y, Yamada K. High-level direct expression of semi-synthetic human interleukin-6 in Escherichia coli and production of N-terminus met-free product. Biotechnology. 1990;8:1036. doi: 10.1038/nbt1190-1036. [DOI] [PubMed] [Google Scholar]
- 18.Reeve VE, Bosnic M, Boehm-Wilcox C, Ley RD. Differential protection by two sunscreens from UV radiation-induced immunosuppression. J Invest Dermatol. 1991;97:624. doi: 10.1111/1523-1747.ep12483006. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura H, Nishimura N, Tohyama C. Immunohistochemical localization of metallothionein in developing rat tissues. J Histochem Cytochem. 1989;37:715. doi: 10.1177/37.5.2703706. [DOI] [PubMed] [Google Scholar]
- 20.Berti E, Cerri A, Tomasini D, Caputo R. Cytokines, cytokine receptors and cytokine antibodies: clinical implications. In: Altmeyer P, Hoffmann K, Stucker M, editors. Skin Cancer and UV Radiation. Berlin: Springer-Verlag; 1997. p. 239. [Google Scholar]
- 21.Schwarz A, Grabbe S, Riemann H, et al. In vivo effects of interleukin-10 on contact hypersensitivity and delayed-type hypersensitivity reactions. J Invest Dermatol. 1994;103:211. doi: 10.1111/1523-1747.ep12393073. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz T, Luger TA. Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B. 1989;4:1. doi: 10.1016/1011-1344(89)80097-1. [DOI] [PubMed] [Google Scholar]
- 23.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:53. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 24.Granstein RD, Sauder DN. Whole-body exposure to ultraviolet radiation results in increased serum interleukin-1 activity in humans. Lymphokine Res. 1987;6:187. [PubMed] [Google Scholar]
- 25.Enk AH, Angeloni AL, Udey MC, Katz SI. Inhibition of Langerhans' cell antigen-presenting function by IL-10. J Immunol. 1993;151:2390. [PubMed] [Google Scholar]
- 26.Enk C, Sredni D, Blauvelt A, Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154:4854. [PubMed] [Google Scholar]
- 27.Enk AH, Katz SI. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992;149:92. [PubMed] [Google Scholar]
- 28.Lots M, Jirik F, Kabouridis P, et al. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988;167:1253. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uyttenhove C, Coulie PG, Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/ plasmacytoma growth factor. J Exp Med. 1988;167:1417. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castells-Rodellas A, Castell JV, Ramirez-Bosca A, Nicolas JF, Valcuende Cavero F, Thivolet J. Interleukin-6 in normal skin and psoriasis. Acta Derm Venereol. 1992;72:165. [PubMed] [Google Scholar]
- 31.Kirnbauer R, Kak A, Schwartz T, et al. IFN-β2 cell differentiation factor 2, or hybridoma growth factor (IL-6) is expressed and released by human epidermal cells and epidermoid carcinoma cell lines. J Immunol. 1992;148:1922. [PubMed] [Google Scholar]
- 32.Schreiber S, Kilgus O, Payer E, et al. Cytokine pattern of Langerhans' cells isolated from murine epidermal cell cultures. J Immunol. 1992;49:3525. [PubMed] [Google Scholar]
- 33.Cumberbatch M, Dearman RJ, Kimber I. Constitutive and inducible expression of interleukin-6 by Langerhans' cells and lymph node dendritic cells. Immunology. 1996;87:513. doi: 10.1046/j.1365-2567.1996.504577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krueger JG, Krane JF, Carter DM, Gottlieb AB. Role of growth factors, cytokines and their receptors in the pathogenesis of psoriasis. J Invest Dermatol. 1990;94:1355. doi: 10.1111/1523-1747.ep12876121. [DOI] [PubMed] [Google Scholar]