Abstract
Cells of the B-cell lineage play an essential part in the immune response, not only as the producers of antigen-specific antibodies, but also as antigen-presenting cells. Unlike T cells, however, the establishment of long-term normal B-cell lines has proved to be exceedingly difficult. In this paper we demonstrate that cell membrane-expressed CD154 (CD40 ligand) is able to support the continual growth of porcine mesenteric lymph node B-cell cultures for more than 4 months without the addition of exogenous cytokines, such as interleukin-4 (IL-4). Addition of IL-4, but not interferon-γ (IFN-γ) or IL-13, to these cultures enhanced proliferation, as, to a lesser extent, did addition of IL-2. Interestingly, however, whilst IFN-γ-supplemented cultures largely consisted of immunoglobulin M (IgM)-positive cells, cultures with IL-13 or IL-4 contained a significantly increased proportion of IgG-positive cells.
INTRODUCTION
Collaboration between B cells and T cells is mediated by both soluble cytokines and contact-dependent cell-surface receptor–ligand interactions. The precise combination of these different signals controls the direction of B-cell responses, resulting in decisions affecting life versus death, memory cell generation and antibody isotype profile. The initial adhesion and subsequent activation of interacting T and B cells requires several distinct sets of cell surface protein–protein interactions.1 For example, interactions between CD40 on B cells and its ligand (CD40L or CD154) on activated T cells in the germinal centre are of crucial importance for the generation of both primary and secondary antibody responses.2,3 Thus the establishment of long-term cultures of normal human B cells,4,5 the induction of memory B cells6 and the immunization of naive mouse B cells7 have been influenced by the ligation of CD40. Furthermore, defective expression of CD154 results in immunological disorders such as X-linked hyper-immunoglobulin M (IgM) syndrome (reviewed in ref. 8). Finally, it is now made clear that CD40 is widely expressed (reviewed in ref. 9) and so CD40–CD154 signalling plays a wider role in the immune system, including involvement of negative selection in the thymus.10 Despite the crucial importance of the CD40–CD154 interaction in immune responses, and with the exception of a recent study on bovine CD40,11 previous studies on this fundamental, and presumably conserved, system have been confined to man and mice.
The pig, as one of the most economically important domestic animals, provides a useful large animal model with an interesting heterogeneity of lymphocyte subpopulations,12 with an easily accessible source of immature B cells in a defined organ, the ileal Peyer’s patch, and with potential utility in human tissue transplantation. In this work therefore we have confirmed that CD40 is expressed by porcine B cells from all tissues and, moreover, demonstrate that long-term porcine B-cell cultures are easily established through CD40–CD154 stimulation, further emphasizing the importance of CD40 ligation in the control of B-cell function and providing a useful model for the exploration of B-cell biology.
MATERIALS AND METHODS
Cells
Thymus, spleen, tonsil, mesenteric lymph nodes (MLN), and ileal Peyer’s patch (IPP) were collected from 1- to 4-week-old outbred piglets (1·6–8 kg), and lymphocytes from these tissues were prepared by teasing the tissues in a Petri dish containing ice-cold Hanks’ balanced salt solution (HBSS). Cells were then passed through nylon mesh and lymphocytes were separated by gradient centrifugation (Nycoprep 1.077 Animal, Nycomed, Oslo, Norway) at 800 gfor 20 min. Contaminating erythrocytes were lysed by brief exposure to distilled water. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of adult pigs by similar gradient centrifugation.
B lymphocytes were purified from various tissues by negative selection using magnetic separation procedures. Cell suspensions were resuspended with mixture of monoclonal antibodies (mAb) against porcine CD3 (PPT3),12 CD8 (11/295/33)13 and macrophage–granulocyte marker sWC3 (74-22-15),13 incubated for 30 min on ice, washed three times with cold HBSS and then incubated with goat anti-mouse immunoglobulin-coated microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 30 min on ice. Once washed with cold MACS buffer [phosphate-buffered saline (PBS) containing 5 mm ethylenediaminetetraacetic acid (EDTA) and 0·5% bovine serum albumin (BSA)], lymphocytes were resuspended with MACS buffer. T cells (CD3+), natural killer (NK) cells (CD8+) and monocytes/macrophages were then depleted from lymphocyte preparations using either AS (for up to 3×107 cells) or BS (for up to 1×108 cells) MACS depletion columns (Miltenyi Biotec) according to the manufacturer’s instructions. In some experiments, B cells from MLN were positively purified using anti-porcine IgM (mouse mAb K52 1C3), anti-CD21(mouse mAb CC51) and goat anti-mouse immunoglobulin-coated microbeads in conjunction with mini-max positive selection columns (Miltenyi Biotec). The purity of B cells was more than 90% as analysed by fluorescence-activated cell sorter (FACS).
CD154 (CD40 ligand)
A soluble murine CD154–CD8α fusion protein-secreting cell line14 (obtained from Dr P. Lane, Basel Institute for Immunology, Switzerland) was maintained in Iscove’s modified Eagle’s minimal essential medium (MEM) (Hyclone, Cramlington, UK) containing 10% fetal calf serum (FCS) and G418 (2 mg/ml, Sigma-Aldrich Co., Dorset, UK). Mouse CD154 or vector-only transfected L929 fibroblast cell lines (K-47 and K-5, respectively)7 were obtained from Professor A. Schimpl (Universität Würzburg, Germany) and maintained in RPMI-1640 medium containing 5% FCS and G418 (0·3 mg/ml).
FACS analysis
The expression of CD40 on porcine B cells from various tissues was analysed by flow cytometry (FACScan, Becton Dickinson, CA) using the ‘lysis II’ program, and gating on small dense lymphocytes. Cells were incubated on ice with phycoerythrin (PE)-labelled goat-anti-porcine immunoglobulin (Southern Biotechnology Associate Inc., Birmingham, AL) and then counterstained with soluble mouse CD154–CD8α, followed by biotin-conjugated rat anti-mouse CD8α monoclonal antibody (Pharmingen, San Diego, CA) and fluorescein isothiocyanate (FITC)-labelled streptavidin (Southern Biotechnology Associate Inc.). Purified B cells, B cells after ligation via CD40 and cultured B cells were stained with the following mAb; anti-IgM (K52 1C3), anti-CD21 (CC51), anti-IL-2R (231 3B2), anti-major histocompatibility complex (MHC) type II (MSA3), anti-CD45 (252 1E4),13 anti-CD3 (PPT3)12 and PE- or FITC-conjugated goat anti-porcine IgG (Southern Biotechnology Associate Inc.). CD80/CD86 expression was examined by recombinant CD152 (CTLA-4)-mIgG fusion protein (Alexis, San Diego, CA) and anti-mouse IgG-PE or anti-mouse IgG-FITC. The degree of cell membrane antigen expression was expressed as mean fluorescence intensity (MFI) after staining and FACS analysis.
Proliferation assays
Iscove’s MEM containing 10% FCS was used for all assays and cell cultures. Lymphocytes from various tissues or purified B cells (2×105 cells/well) were cultured with or without soluble CD154–CD8α, γ-irradiated (5500 rads) cell membrane expressing CD154 (K-47 cells; 2×103 cells/well), or γ-irradiated (5500 rads) negative control cells (K-5 cells; 2×103 cells/well) in 96-well plates at 37° for various periods. In some experiments, recombinant porcine IL-2 (rpIL-2),15 recombinant human IL-2 (rhIL-2, Genzyme, Cambridge, MA), recombinant human IL-4 (rhIL-4, Genzyme), recombinant porcine interferon-γ (rpIFN-γ, from Dr B. Charley, INRA, Jouy-en-Josas, France) or recombinant human IL-13 (rhIL-13, Endogen, Cambridge, MA) was added. [3H]TdR (0·2 μCi/well) was added to each well 16 hr prior to harvest and incorporation was determined by liquid scintillation counting (1205 Betaplate, Wallac, Turku, Finland). Each individual group consisted of triplicate cultures and the standard deviation was never more than 10% of the mean.
Measurement of apoptosis
Positively purified MLN B cells were incubated with goat anti-porcine immunoglobulin for 30 min on ice, washed with medium, then cultured at 37° with or without CD154 for various periods. Apoptosis of activated B cells was assessed by FACS analysis of size/granularity and DNA/content. For the latter, cells were ethanol fixed, RNase digested and stained with propidium iodide (Sigma) as described.16
Long-term porcine B-cell cultures
Purified B cells (1·5×106 cells/ml) from MLN were cocultured with γ-irradiated (5500 rads) K-47 cell monolayer (expressing CD154) in 25 cm2 flasks at 37°. Each week after the culture, viable B cells were recovered by gradient centrifugation (Nycoprep, 800 g, 20 min), and B cells (adjusted roughly 1·5×106 cells/ml) were transferred to new flasks containing a monolayer of γ-irradiated K-47 cells.
Characterization of the B-cell cultures
The CD154-MLN B-cell cultures were supplemented with rhIL-2 (10 U/ml), rhIL-4 (5 ng/ml), rhIL-13 (10 ng/ml), rpIFN-γ (10 ng/ml) for 1 week. Cultures with and without cytokines were assayed for viability by trypan blue exclusion, for secreted immunoglobulin by enzyme-linked immunosorbent assay (ELISA), and for immunoglobulin isotype by immunohistochemical staining of fixed cytospin samples. In brief, cytospin samples were air-dried, fixed with ice cold acetone for 10 min. Endogenous peroxidase activity was suppressed by Immuno Pure peroxidase suppresser (Pierce, Rockford, IL) and intracellular immunoglobulins were detected by mAb K52 1C3 (anti-IgM), K61 1B4(anti-IgA), K138 4C12 (anti-IgG1/G2)(these mAb were obtained from Dr C. Stokes, Bristol University, UK). Bound mAb to the different porcine immunoglobulin isotypes were revealed by staining with Vectastain Elite ABC kit (Vector, Burlingame, CA) with 3,3′-diaminobenzidine (DAB) substrate kit (Vector) according to the manufacturer’s instructions. Samples were then counterstained with haematoxylin (Vector).
In order to detect secreted immunoglobulin, Nunc-Immunoplates (Nalge Nunc International, Rochester, NY) were coated with affinity purified goat anti-porcine immunoglobulin (10 μg/ml, 50 μl/well, Southern Biological Associate Inc.) in carbonate–bicarbonate buffer (pH 9·6) for 1 hr at 37°. The plates were washed with PBS/Tween (PBS containing 0·02% Tween-20). Samples or standard purified porcine immunoglobulin (Sigma) were diluted in PBS/Tween containing 3% ovalbumin (OVA, Sigma) and added to the plate (50 μl/well). After 2 hr incubation at 37°, plates were washed three times with PBS/Tween. For total porcine immunoglobulin detection, horseradish peroxidase-conjugated goat anti-porcine immunoglobulins (Southern Biological Associate Inc.) were added to each well (50 μl/well) and incubated for a further 1 hr at 37°. Plates were washed five times with PBS/Tween, substrate (OPD, Sigma) was added and optical density (OD) was measured at 495 nm.
RESULTS
Expression of CD40 and the effect of CD40 ligation on porcine B cells
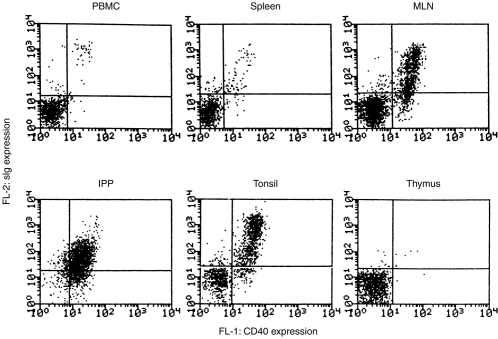
Expression of CD40 on porcine B cells was demonstrated by FACS analysis. The majority of surface immunoglobulin (sIg) -positive porcine lymphocytes (B cells) from peripheral blood, spleen, MLN and tonsil expressed CD40, but thymocytes did not (Fig. 1). The expression of CD40 on IPP B cells was lower (MFI=21) than B cells from other lymphoid tissues (MFI=42) but, as was clear from the analysis, most IPP lymphocytes were sIg+ and thus B cells.
Figure 1.
Expression of CD40 on porcine B cells. Porcine lymphocytes from blood (PBL), spleen, mesenteric lymph nodes (MLN), tonsil, ileal Peyer’s patch (IPP) and thymus were analysed for expression of CD40 by staining with soluble CD154 (CD40 ligand)–mCD8α fusion protein, and biotin conjugate-monoclonal anti-CD8, then FITC-labelled streptavidin. The B cells were then revealed by counterstaining with PE coupled anti-porcine immunoglobulin.
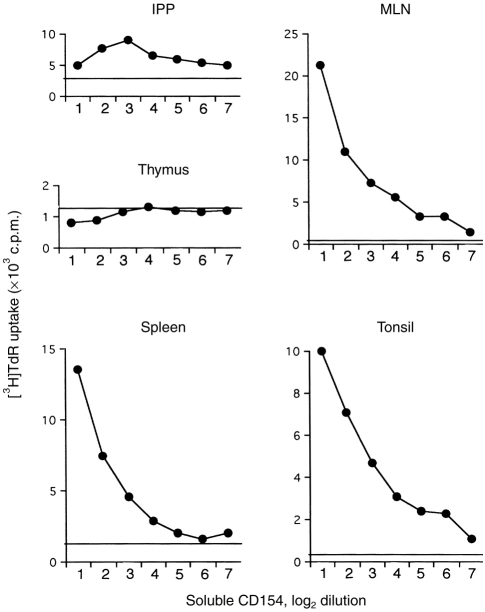
Next, we demonstrated the functional capacity of CD40 by stimulating proliferation of B cells in vitro with soluble CD154 (Fig. 2). Lymphocytes from MLN were efficiently stimulated by soluble CD154 in a dose-dependent manner, lymphocytes from IPP were less active, and thymocytes not at all. As an alternative, B-cell activation by CD40 ligation was also demonstrated by increased cell size (data not shown) and increased expression of IL-2R, CD80/CD86, sIg and MHC II (Table 1).
Figure 2.
Induction of B-cell proliferation by soluble CD154. Negatively purified B cells from ileal Peyer’s patch (IPP), mesenteric lymph node (MLN), spleen and tonsil were cultured with serial dilutions of soluble CD154 secreted by cell line (log 2 dilution) for 3 days. Proliferation was assessed by [3H]TdR uptake and data are shown as c.p.m. The background counts (BG) are shown as horizontal lines. A thymocyte suspension included as a negative control. The results are the average of triplicate cultures where the standard deviation was<10% of the mean.
Table 1.
Phenotypes of purified porcine MLN B cells before and after cultivation with CD154 antigens
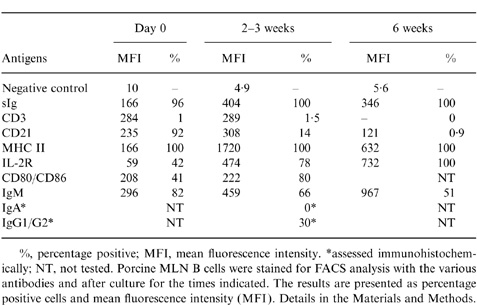
%, percentage positive; MFI, mean fluorescence intensity.
*assessed immunohistochemically; NT, not tested. Porcine MLN B cells were stained for FACS analysis with the various antibodies and after culture for the times indicated. The results are presented as percentage positive cells and mean fluorescence intensity (MFI). Details in the Materials and Methods.
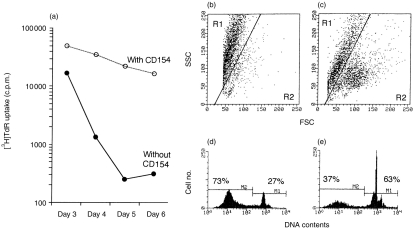
Stimulation of porcine MLN B cells with sIg resulted in stimulation of DNA synthesis, demonstratable by [3H]TdR uptake (Fig. 3a), followed by a rapid reduction of DNA synthesis and entry into apoptosis (Fig. 3b, d). Addition of soluble CD154 to these cultures however, resulted in sustained thymidine incorporation (Fig. 3a) and a considerably higher number of viable, non-apoptotic cells, by day 7 post-stimulation (Fig. 3c,e).
Figure 3.
Soluble CD154 rescues activated B cells from apoptosis. Positively (anti-immunoglobulin) purified MLN B cells were cultured with anti-immunoglobulin with or without soluble CD154, and [3H]TdR uptake was measured daily. (a) After 6 days the cultures were assessed for apoptosis by either SSC/FSC analysis (b,c) or DNA analysis (d.,e) in the absence (b,d) or presence (c,e) of soluble CD154. The results are the average of triplicate cultures where the standard deviation was < 10% of the mean.
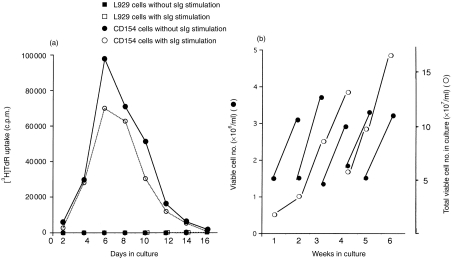
Cell membrane-expressed CD154 provided a potent stimulus for purified MLN B cells with or without anti-immunoglobulin treatment (Fig. 4). Optimum DNA synthesis occurred at 6 days post-stimulation, after which there was a progressive decline. Stimulation of B cells with anti-immunoglobulin in addition to membrane-expressed CD154 marginally decreased the thymidine incorporation, whilst B cells cultured with the negative control fibroblast cell line (K-5) did not proliferate (Fig. 4a).
Figure 4.
Cell membrane expressed CD154 is a very effective mitogen for negatively purified B cells. (a) Proliferation of MLN B cell was induced by cell membrane expressed CD154, but not by vector control transfected L929 cells, with or without stimulation via sIg. The results are the average of triplicate cultures where the standard deviation was<10% of the mean. (b) Growth of MLN B cells is supported by cell membrane expressed CD154. Proliferating viable B cells were separated from cultures by gradient centrifugation each week, cell number was counted and then B cells (≈1·5×106 cells/ml) were transferred to new flasks containing γ-irradiated cell membrane expressed CD154 cells. Growth of B-cell number (viable cells only) over 6 weeks is shown (b). Closed circles show the cell number per ml in culture and open circles show the total viable cell number in the cultures.
Establishment of long-term B-cell cultures with cell membrane expressed CD154
As optimum stimulation of proliferation of B cells with membrane-expressed CD154 occurred after 1 week of stimulation, viable cells were recovered at this time-point and were transferred to new flasks containing freshly prepared (K-47) cells expressing CD154. This subculturing procedure when repeated, resulted in a weekly doubling of B cells with, for example as shown in Fig. 4b, an increase from 1·5×107 to 1·2×108 viable B cells in 3 weeks. At the same time, however, these cultures also contained a large number (20–50%) of dead cells, indicating a rapid turnover rather than a prolonged life span of individual B cells. The CD40 dependency of the culture was absolute as neither the negative transfectant cells (K-5), nor the soluble CD154 alone, were able to maintain the B cells (data not shown). Even in the presence of the CD154-expressing transfectant and with the weekly subculturing procedure, the cultured B cells eventually lost their response to CD154 and, after 3–4 months, ceased to grow.
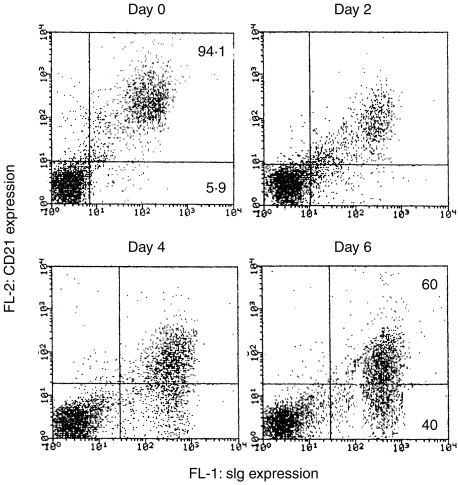
All the cultured cells were sIg+, CD45+, MHC Class II+ and IL-2R+. The proportion of sIgM+ cells, however, decreased to 50–60% after 6 weeks culture (Table 1), and there was an almost total loss of CD21 expression after 6 weeks’ culture. As the loss of CD21 expression by activated or early terminally differentiated B cells has been reported in other animals,17 we looked in detail at CD21 expression on porcine B cells at different times after combined stimulation with anti-sIg and CD154 (Fig. 5). Before stimulation via sIg and CD154, the majority of MLN B cells (94·1%) expressed CD21 with a relatively high MFI (429). After stimulation, the expression of CD21 on sIg+ cells was gradually reduced from a starting level of 94·1% positive cells (MFI of 429) to 60% positive (MFI of 83) by day 6. In contrast, the expression of sIg increased from MFI=166 at day 0 to 404 at day 6 of activation.
Figure 5.
Reduced expression of CD21 by activated porcine B cells. Total MLN lymphocytes were stimulated via sIg and cocultured with cell membrane expressed CD154 cells, and the expression of CD21 was analysed by staining with mouse mAb anti-CD21 and PE-goat anti-mouse immunoglobulin. B cells were counterstained with FITC anti-pig immunoglobulin.
Effect of cytokines on stimulated B-cell culture
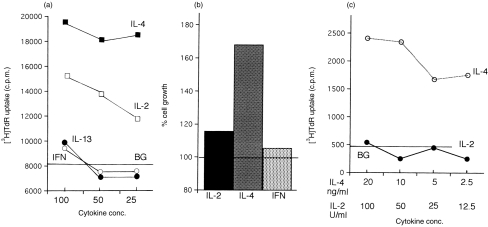
Established CD154-dependent B-cell lines were incubated with relevant, available cytokines immediately after the weekly subculture and then assessed by thymidine uptake and cell counting 7 days later. A costimulatory effect of rhIL-4 was clearly demonstrated by both [3H]TdR uptake (Fig. 6a) and cell growth (Fig. 6b). In the absence of cell membrane-expressed CD154, rhIL-4 was able to stimulate DNA synthesis of MLN B-cell cultures (Fig. 6c), but only for short periods. Although less effective than rhIL-4, rhIL-2 also costimulated [3H]TdR uptake and cell growth (Fig. 6a,b). Unlike IL-4, however, IL-2 was unable to stimulate MLN B-cell cultures without CD154 (Fig. 6c). Both rhIL-13 and rpIFN-γ were without a significant effect on MLN B-cell stimulation or cell growth (Fig. 6a,b).
Figure 6.
Effect of cytokines on negatively purified MLN B cell cultures. The costimulatory effect of cytokines IL-2 (25–100 U/ml), IL-4, IL-13, IFN-γ (25–100 ng/ml) on CD154-MLN B cell culture was examined after 7 days treatment by [3H]TdR uptake (a). CD154 alone (without cytokine) gave an incorporation of 8170±829 c.p.m. (shown in the figure as a horizontal line). IL-4 enhanced MLN B-cell growth with CD154 (b). Figure shown as percentage cell growth (B-cell number in CD154-MLN B-cell culture with cytokine/B-cell number with CD154 alone×100). IL-4 but not IL-2 was able to induce MLN B-cell proliferation without CD154 (c). Background count (without CD154 nor cytokine) shown as a horizontal line (480 c.p.m.). The results are the average of triplicate cultures where the standard deviation was<10% of the mean.
Finally, the effect of cytokines on the profile of immunoglobulin isotypes secreted by CD154-MLN B-cell cultures was assessed. The B-cell cultures were first established for 3 weeks and then incubated for a further week with or without added cytokines, prior to the measurement of the immunoglobulin isotype profile. In the absence of exogenous cytokine, 50% of the cultured cells were IgM-positive, 30% were IgG1/G2-positive, but no IgA-positive cells were observed. Addition of IL-4 or IL-13 for 1 week increased the number of IgG1/IgG2-positive cells to 42–52% with a corresponding reduction of IgM-positive cells to 35–37%. In contrast, either IFN-γ or IL-2 reduced the number of IgG1/G2-positive cells to 5–18% and increased IgM-positive cells to 75%. Various combinations of cytokines were tested and showed no clear cytokine-dominated effect, apart from IL-2/IL-4, which marginally induced detectable numbers of IgA-positive cells (7%).
Anti-immunoglobulin stimulated MLN B cells cultured with rhIL-2 produced 80–140 ng/ml of porcine immunoglobulin after a 7-day cultured period, however, when CD154 was present, more than 1400 ng/ml porcine immunoglobulin was secreted in the culture over the same period.
DISCUSSION
The work confirms and extends the concept of a fundamental and conserved function for the CD40–CD154 ligand–counterligand interaction in B-lymphocyte physiology. In extending our enquiries to the pig system, we have observed that porcine B-cell lines are surprisingly easy to establish, and this may provide a useful tool, unavailable in mouse and man, to explore later events in the control of B-cell nature and development.
The B lymphocyte occupies a central role in immune responses, serving not only as the precursor of antibody-secreting cells, but also as effective antigen-presenting cells. It is hardly surprising therefore that there have been many attempts to produce permanent B-cell lines but, unlike corresponding efforts with T cells, these have largely come to nothing. Indeed, in the only successful work to date, using the procedure that formed the starting point of our work, the human B-cell lines that were grown displayed an obligatory requirement of IL-4.4 Our porcine B-cell lines, on the other hand, proliferated in the absence of this growth factor.
The interspecies conservation of the CD40–CD154 functional interaction is indicated by a similar mitogenic activity of CD154 for both human and murine B cells,17 and the cross-reactivity of these recombinant CD154 molecules with ovine18 and bovine19 B cells. The biological consequence of CD40 ligation was also similar in pigs, resulting in induction of B-cell growth and proliferation (Fig. 2,Fig. 4), rescue of activated B cells from apoptosis (Fig. 3), and a differential immunoglobulin class profile of the resulting differentiated cells depending on the cytokine costimulus. This degree of conservation between pigs and man is exceptional. For example, only 8% of mAb to human CD antigen cross-react with pigs,20 and there is almost none with any anti-mouse CD antigens. Furthermore, generation of the immunoglobulin variable region repertoire in pigs, ruminants, rabbits and chickens proceeds via gene conversion21,22 in a specialized B-cell organ, the IPP (in pigs and ruminants), bursa of Fabricius (in chickens) and appendices (in rabbits); as opposed to mice and man, where B cells originate in the bone marrow and the antibody repertoire relies to a large extent on multiple germ line variable regions. Finally, pigs, unlike mice and man, do not express IgD.21 All of this serves to emphasize the basic, essential and conserved role of CD40 in B-cell biology although many variations in other essential components of the immune response do occur between different animal species.
In man, CD40 is expressed from early stages of B-cell ontogeny, being detected on CD34+ progenitor cells,23 CD10+, CD19+, sIg-B cell precursors,23,24 and on fetal liver CD19+ pro-B cells.25 Studies in vitro show that these human B-cell precursors can be induced into proliferation and high levels of CD23 expression by CD40 ligation but without a consistently observed further differentiation.25,26 Ligation of pre-B cell CD40, on the other hand, induces sIg isotype switch and immunoglobulin production in the presence of IL-4.27 These observations indicate the possible involvement of CD40 in the early stages of B-cell development and differentiation in vitro. Interestingly, CD40-signalling may also be involved in sheep IPP B-cell differentiation,18 where immature B-cell selection is thought to occur without antigen or T-cell involvement.18,28 All of this and our recent demonstration of a role for CD40 ligation in the selection and differentiation of immature porcine B cells (J.K. Andersen et al. 1998, unpublished data) once again emphasizes a profound role for CD40 in B-cell biology. Thus exploration of the consequences of CD40-CD154 ligation in the porcine B-cell system may provide a unique and useful tool for studies of B-cell development in health and disease.
The long-term culture of CD40-activated human B cells requires exogenous IL-4.4,5 In contrast, porcine B-cell lines propagated by CD40-ligation alone continued to proliferate and survive for more than 4 months which is far longer than the human B cells cultured with both CD154 and IL-4. Further addition of IL-4, however, did induce a stronger and more prolonged proliferation and growth of porcine B cells. Thus signalling via porcine CD40 may have stronger and more prolonged biological effects in the pig than in man. Such minor biological differences may have an important implication for xenotransplantation strategy. For example, as CD40 is now recognized to be expressed in cells other than B cells, indeed in endothelial cells,29 our demonstration that ligation of CD40 induces CD80/86 expression in activated B cells, may be relevant.
Due to the limited availability of reagents, only a restricted study of the combined effect of CD40, anti-immunoglobulin and cytokine stimulation was possible. Nonetheless, it is clear that co-operative effects do occur in the development of porcine B cells, both in quantitative and qualitative terms. Thus, ligation of CD40 with CD154 greatly increases the amount of secreted immunoglobulin. In addition, IL-4, and to a lesser extent IL-13, decreased IgM expression whilst stimulating IgG expression in porcine B-cell cultures stimulated with CD40. Addition of IL-2 and IFN-γ to these cultures, on the other hand, resulted principally in IgM expression, with little IgG. It will be interesting to extend these studies as further reagents inevitably appear. It will also be important to systematically explore these combinations of conditions with porcine B cells representative of the major, definable stages of B-cell development, in particular, the readily accessible specialized IPP, where immature B cells are generated.
REFERENCES
- 1.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 2.Durie FH, Foy TM, Masters SR, Laman JD, Noelle RJ. The role of CD40 in the regulation of humoral and cell-mediate immunity. Immunol Today. 1994;15:406. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 3.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39–CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, De Paoli P, Vallé A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 5.Rousset F, Peyrol S, Garcia E, et al. Long-term cultured CD40-activated B lymphocytes differentiate into plasma cells in response to IL-10, but not IL-4. Int Immunol. 1995;7:1243. doi: 10.1093/intimm/7.8.1243. [DOI] [PubMed] [Google Scholar]
- 6.Arpin C, Déchanet J, Van Kooten C, et al. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 7.Wohlleben G, Gray D, Scimpl A. In vitro immunization of naive mouse B cells: Establishment of IgM secreting hybridomas specific for soluble protein or hapten from B cell cultured on CD40 ligand transfected mouse fibriblasts. Int Immunol. 1996;8:343. doi: 10.1093/intimm/8.3.343. [DOI] [PubMed] [Google Scholar]
- 8.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 9.Van Kooten C, Banchereau J. CD40–CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 10.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 11.Hirano A, Brown WC, Estes DM. Cloning, expression and biological function of the bovine CD40 homologue: role in B-lymphocyte growth and differentiation in cattle. Immunology. 1997;90:294. doi: 10.1046/j.1365-2567.1997.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Parkhouse RME. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology. 1996;89:76. doi: 10.1046/j.1365-2567.1996.d01-705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saalmüller A. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352. doi: 10.1016/S0167-5699(96)90273-X. [DOI] [PubMed] [Google Scholar]
- 14.Lane P, Brocker T, Hubele B, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993;177:1209. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inumaru S, Denyer MS, Takamatsu H, Ryan M, Parkhouse RM.E. Expression and purification of biologically active recombinant porcine interleukin 2. Vet Immunol Immunopathol. 1993;35(Suppl.):120. [Google Scholar]
- 16.Sherwood SW, Schimke RT. Cell cycle analysis of apoptosis using flow cytometry. In: Schwartz LM, Osborne BA, editors. Methods in Cell Biology Cell Death. Vol. 46. New York: Academic Press; 1995. p. 77. [DOI] [PubMed] [Google Scholar]
- 17.Spriggs MK, Armitage RJ, Strockbine L, et al. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J Exp Med. 1992;176:1543. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griebel P, Ferrari G. CD40 signalling in ileal Peyer’s patch B cells: implication for T cell-dependent antigen selection. Int Immunol. 1995;7:369. doi: 10.1093/intimm/7.3.369. [DOI] [PubMed] [Google Scholar]
- 19.Mukwedeya DT. University of Bristol; 1995. Bovine B cells: heterogeneity, activation and targeted antigen presentation. PhD. thesis. [Google Scholar]
- 20.Sopp P, Redknap L, Howard C. Cross-reactivity of human leukocyte differentiation antigen monoclonal antibodies on porcine cells. Vet Immunol Immunopathol. 1998;60:403. doi: 10.1016/s0165-2427(97)00114-1. [DOI] [PubMed] [Google Scholar]
- 21.Butler JE, Sun J, Kacskovics I, Brown WR, Navarro P. The VH and CH immunoglobulin genes of swine: implications for repertoire development. Vet Immunol Immunopathol. 1996;54:7. doi: 10.1016/s0165-2427(96)05680-2. [DOI] [PubMed] [Google Scholar]
- 22.Parng C-L, Hansal S, Goldsby RA, Osborne BA. Gene conversion contributes to Ig light chain diversity in cattle. J Immunol. 1996;157:5478. [PubMed] [Google Scholar]
- 23.Saeland S, Duvert V, Caux C, et al. distribution of surface-membrane molecules on bone marrow and cord blood CD34+ hematopoietic cells. Exp Hematol. 1992;20:24. [PubMed] [Google Scholar]
- 24.Uckun FK, Gajil-Peczalska K, Myers DE, Jaszcz W, Haissig S, Ledbetter JA. Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgikin’s lymphoma cells. Blood. 1990;76:2449. [PubMed] [Google Scholar]
- 25.Sealand S, Buvert V, Moreau I, Banchereau J. Human B cell precursors proliferate and express CD23 after CD40 ligation. J Exp Med. 1993;178:113. doi: 10.1084/jem.178.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renard N, Duvert V, Blanchard D, Banchereau J, Saeland S. Activated CD4+ T cells induce CD40 dependent proliferation of human B cell precursors. J Immunol. 1994;152:1693. [PubMed] [Google Scholar]
- 27.Punnonen J, Aversa G, Vries JE. Human pre-B cell differentiate into Ig-secreting plasma cells in the presence of IL-4 and activated CD4+ T cells or their membranes. Blood. 1993;82:2781. [PubMed] [Google Scholar]
- 28.Reynolds JD, Morris B. The effect of antigen on the development of Peyer’s patches in sheep. Eur J Immunol. 1984;14:1. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- 29.Maher SZ, Karmann K, Min W, Hughes CC.W, Pober JS, Bothwell AL.M. Porcine endothelial CD86 is a major costimulator of xenogenic human T cells. J Immunol. 1996;157:3838. [PubMed] [Google Scholar]