Abstract
Dysregulation of both B- and T-cell responses is observed in leprosy. Immunoglobulin G1 (IgG1) and IgG3 antibody subclasses are selectively elevated towards the lepromatous or disseminated form of the disease accompanied by a depression of T-cell responses. T-cell and macrophage cytokines influence antibody class switching, differentiation and proliferation of B cells. To understand the dynamic nature of the immune response in leprosy, we examined the relationship between circulating Mycobacterium leprae-specific antibodies and secreted cytokines [interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-5, IL-10, IL-6, tumour necrosis factor-α (TNF-α) and granulocyte–macrophage colony-stimulating factor (GM-CSF)] in leprosy patients (19 lepromatous patients; 25 tuberculoid patients) and their exposed household contacts (HC=14) in response to M. leprae antigens. Paired comparison revealed a highly significant negative correlation between IFN-γ and IgG (P=0·016), IgG1 (P<0·001) and IgG3 (P=0·007) antibodies. No significant relationship was observed with other T-cell cytokines (IL-2, IL-5 and IL-10). These results strongly suggest that IFN-γ may play a role in down-regulating antigen-specific IgG1 and IgG3 antibodies. Among the macrophage cytokines, TNF-α and GM-CSF which have not been shown to play a role in B-cell activation were positively associated with IgG1 (TNF-α, P=0·0005; GM-CSF, P=0·001) and IgG3 (TNF-α, P=0·001; GM-CSF, P=0·021) antibodies. Since macrophages have high-affinity Fc receptors for IgG1 and IgG3, it is possible that antigen uptake via these receptors may influence cytokine expression of TNF-α, a key modulator of disease pathogenesis in mycobacterial diseases. We are currently investigating the role of Fc receptors on activated macrophages, in expression of pro-inflammatory cytokines in mycobacterial diseases.
INTRODUCTION
Leprosy with its defined spectrum of clinical disease has been shown to be directly related to dysregulation of several immune parameters.1–3 The disseminated or lepromatous form of the disease is characterized by depressed T helper type 1 (Th1) responses and augmented B-cell responses, while tuberculoid leprosy, which is the more localized form of the disease, shows Th1 responses [including interferon-γ (IFN-γ) secretion] to Mycobacterium leprae-specific antigens and decreased antibody responses.4,5 In leprosy patients, several isotypes, including immunoglobulin M (IgM), IgG and IgA antibodies, are raised towards the lepromatous pole.3 Isotype switching from IgM to IgG and IgA requires both T cells and macrophage cytokines.6 IgG is composed of four subtypes, namely IgG1–IgG4, which are differentially regulated by T-cell cytokines, in both mouse and humans.6 In leprosy, IgG1 and IgG3 antibodies are selectively augmented in patients, particularly in lepromatous disease, and show a highly significant correlation with bacterial load.7 IgG1 and IgG3 antibodies are complement-fixing antibodies and markers of the pro-inflammatory response,8 and may participate in erythema nodosum leprosum (ENL), an immune complex-mediated reaction observed towards the lepromatous pole of the disease.9,10 It has recently been shown that cytokines, such as interleukin-10 (IL-10), secreted by both activated macrophages and T cells, may play a direct role in the switching of IgG1 and IgG3 human B cells.11 The role of IFN-γ secreted by activated T cells is unclear. In the murine system, IFN-γ has been demonstrated to up-regulate IgG2a12 while in the human system it has been shown to be a potent inhibitor of B-cell responses. Because the disease spectrum of leprosy correlates with a defined immune spectrum of T- and B-cell responses, we have further analysed the dynamic nature of immune response in leprosy by extending the analysis to cytokines secreted by antigen-stimulated T cells and macrophages and circulating M. leprae-specific IgG subclass antibodies.
MATERIALS AND METHODS
Study subjects
Newly diagnosed leprosy patients presenting at the Marie Adelaide Leprosy Centre (MALC) were recruited to our studies and have been described in detail elsewhere.13 Patients are diagnosed clinically as well as histologically on a 4-mm punch biopsy taken from the edge of an active lesion.13 Forty-four patients from across the leprosy spectrum [polar lepromatus/borderline lepromatus (LL/BL), n=19; borderline tuberculoid/polar tuberculoid (BT/TT), n=25] who had not been treated for leprosy previously were included in the study. Healthy controls included household contacts of leprosy patients with lepromatous disease (HC, n=14) to ensure recent exposure. Ethical approval was obtained from both the Aga Khan University and the MALC Human Rights Protection Committees. Written/oral consent as appropriate was obtained from both patients and control groups.
Antigens
Mycobacterium leprae sonicate (MLSON) Lot CD197 was obtained through the courtesy of Dr J. Colston, National Institute of Medical Research, UK.
Antisera
Five millilitres of blood collected from leprosy patients was allowed to separate overnight at 4°. Serum was removed and centrifuged at 400 g for 15 min; the clear supernate was distributed in small aliquots and frozen at −70° before use.
Assay of lymphocyte blastogenesis
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood (30 ml) by density sedimentation over Ficoll–Hypaque. Cells were washed three times with medium (RPMI-1640; Bio Whittaker, Walkersville, MD). Cells were counted and suspended in complete medium (RPMI-1640 with 2 mm l-glutamine, 100 mg/ml of gentamicin, 15 mm HEPES and 20% autologous human plasma). Two hundred thousand cells per well were placed in round-bottomed microtitre tissue culture plates (Flow Laboratories, Irvine, UK). The M. leprae sonicate (MLSON; 10 μg/ml), was added to triplicate wells for each donor. Control wells received medium alone. The cultures were incubated for 5 days in 5% CO2 at 37°. One microcurie of [3H]thymidine (specific activity 6·7 Ci/mmol; Amersham Laboratories, Bucks, UK) was added to each culture well for the final 18 hr. Cells were harvested after 18 hr with a PHD cell harvester (Cambridge Technology, Cambridge, MA) and [3H]thymidine incorporation was measured in a scintillation counter. Results were expressed as mean counts per minute (c.p.m.) of the triplicates. Spontaneous incorporation of [3H]thymidine in cultured cells ranged between 500 and 1000 c.p.m.
Assay for T-cell and macrophage cytokines in culture supernatants of stimulated PBMC
Supernatants were collected from stimulated cells after 5 days for determination of T-cell cytokines (IFN-γ, IL-2, IL-5 and IL-10) and after 48 hr for determination of cytokines secreted by macrophages [tumour necrosis factor-α (TNF-α), IL-6 and granulocyte–macrophage colony-stimulating factor (GM-CSF)]. TNF-α, IL-6 and IL-2 were obtained from Quantitakine (Minneapolis, MN); IFN-γ and IL-5 from Pharmingen (San Diego, CA); IL-10 from Predicta (Cambridge, MA) and GM-CSF from Genzyme (Cambridge, MA). All cytokines were detected by enzyme-linked immunosorbent assay (ELISA) -based assays. The assays were carried according to the manufacturer’s recommendation. Supernatants were appropriately diluted where necessary to obtain values within the detection range.
Reagents, monoclonal antibodies and conjugates
Monoclonal antibodies specific for human IgG subclasses were: HP 6001(anti-IgG1), HP 6002 (anti-IgG2), HP 6047 (anti-IgG3), HP 6023 (anti-IgG4) and HP 6029 (anti-IgE) prepared at the Centre for Disease Control, (Atlanta, GA) were a gift from Dr Reimer. The specificity evaluation and performance characteristics of these antibodies are described in detail elsewhere.14,15 Goat anti-human IgG (Fc-specific) and goat anti-mouse IgG (H+L chain-specific), conjugated to alkaline phosphatase were commercially obtained (Jackson Immuno Research Laboratories, Westgrove, PA) and diluted according to the manufacturer’s recommendations. Preparation of purified rabbit anti-human IgE has been described in detail previously16 and were obtained by immunizing rabbits with the Fc fragments of human IgE myeloma, affinity-purified over an IgE Sepharose column and rendered epsilon-Fc-specific by sequential passage over affinity columns of insolubilized IgG and F(ab′)2 fragments of human myeloma IgE.
Quantification of IgG and IgG subclasses and IgE to MLSON
IgG and IgG subclasses were quantified using an ELISA-based assay as previously described.7 Briefly, Immulon 4 plates were coated with 100 μl of each antigen at 1 μg/ml in carbonate buffer pH 9·6 for 2 hr at 37° and then overnight at 4°. Phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) was added for 2 hr at 37° to block free sites. Then, 100 μl of sera diluted in PBS containing 0·05% Tween-20 and 1·0% BSA were added and incubated for 2 hr at 37° and then overnight at 4°. For IgG antibodies, goat anti-human IgG conjugated to alkaline phosphatase was added for 2 hr at 37°. For IgG subclasses and IgE, monoclonal antibodies specific for each of the IgG subclasses and IgE were added at saturation concentrations of 1:1000 for HP 6001, HP 6002 and HP 6047 and further incubated overnight at 4°. Alkaline phosphatase-labelled goat anti-mouse IgG was then added and incubated for 2 hr at 37°. The plates were finally developed with alkaline phosphatase substrate. Each incubation was followed by three washes with PBS containing 0·05% Tween-20 to remove unbound protein. All test sera were run at a minimum of three dilutions and the activity was expressed as [optical density in the linear range×dilution] of the sera. In each assay a reference pool containing high titre of IgG subclass antibodies was used as a calibrator for interassay variability. To control for non-specific binding, pooled sera from normal healthy donors were used as the negative control for each assay.
Statistics
Statistical analysis was done on an Apple Macintosh microcomputer using statview™ software packages. Paired comparison using the two-tailed Fisher exact test was used to determine the relationship between antibody and cytokine responses.
RESULTS
Immune characteristics of the study groups
A total of 44 untreated leprosy patients were tested for lymphocyte proliferation, cytokine secretion and IgG antibody subclass responses to MLSON, of which 19 were multibacillary lepromatous (L=LL, BL, BB) and 25 were paucibacillary tuberculoid (T=BT, TT, Indeterminate). In addition 14 household contacts (HC) of untreated active cases of patients with lepromatous leprosy were also included as exposed but healthy controls. Figure 1 shows the lymphocyte proliferation in MLSON –stimulated cultures after deducting responses of unstimulated cultures (Δc.p.m.). The leprosy patients showed the expected antigen-specific T-cell response profile, with high responses in the tuberculoid group and depressed antigen-specific responses in the lepromatous group (P<0·0001). A parallel pattern of IFN-γ secretion in response to MLSON was observed (Fig. 1b). This typical pattern of response was therefore reassuring to analyse the dynamic relationship between various cytokines and circulating antibodies across the infection/disease spectrum.
Figure 1.
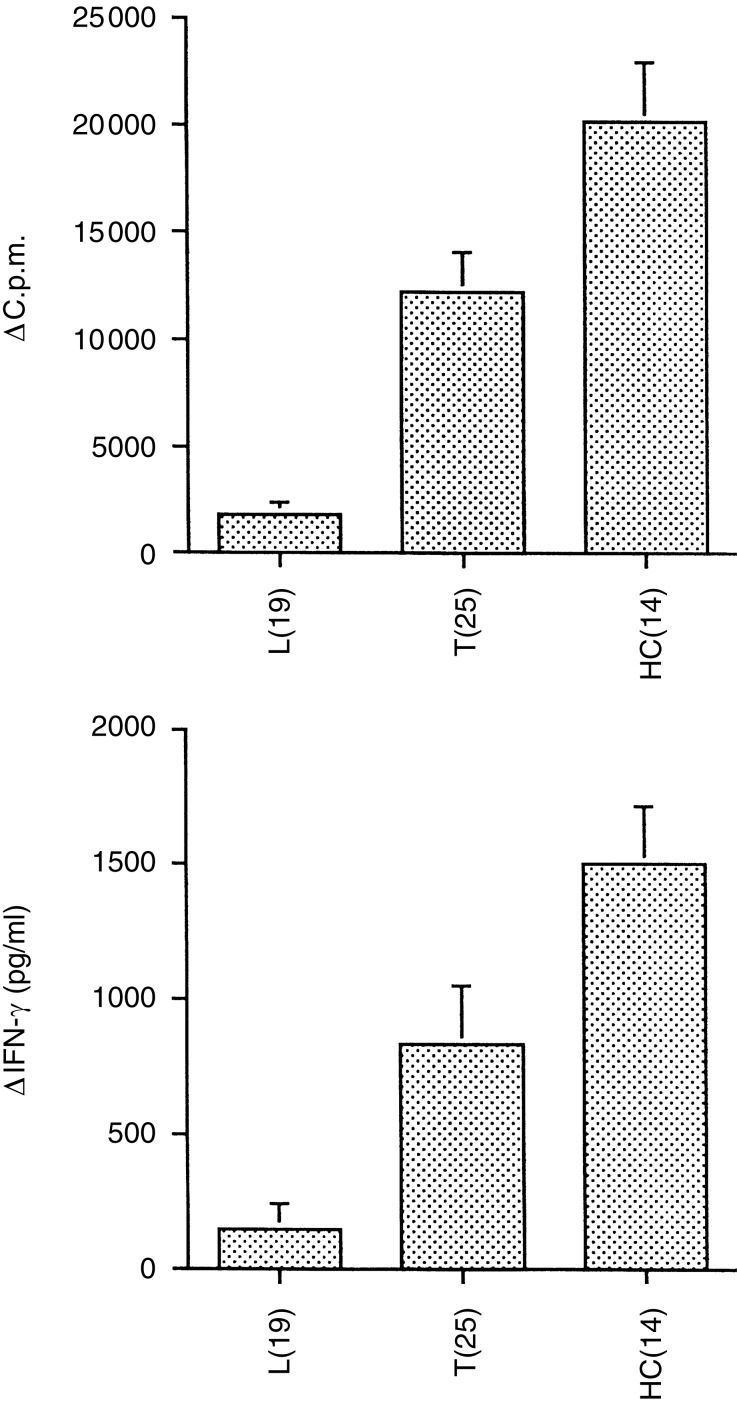
Immune profile of leprosy patients and exposed healthy household contacts (HC). Lymphocyte proliferative responses (10 μg/ml) (a) and secretion of IFN-γ (b) were detected in supernatants after 5 days of M. leprae antigen stimulation of PBMCs. Patient groups were lepromatous and borderline lepromatous (L), borderline tuberculoid and tuberculoid (T) type of leprosy and exposed healthy household contacts (HC). The number of individuals tested in each group is indicated in brackets. (a) Lymphoproliferative responses as mean Δc.p.m. of the group±1SEM and (b) IFN-γ responses as mean pg/ml±1SEM.
Relationship of MLSON-induced IFN-γ and MLSON-specific IgG and IgG subclass antibodies in leprosy patients and M. leprae-exposed individuals
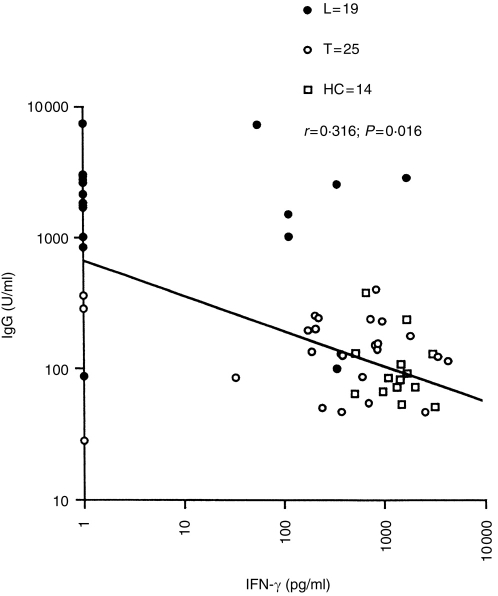
We initially analysed the relationship of IFN-γ and antigen-specific IgG antibodies. Figure 2 shows the scatter plot and regression line for IgG antibodies specific for MLSON and IFN-γ secretion in MLSON-stimulated PBMCs from across the exposed and disease spectrum to understand the dynamics of immune responses in relation to antigen burden. IgG antibodies showed a strong negative correlation with IFN (r=−0·316; P=0·016) from MLSON-stimulated PBMCs. This may suggest that IFN itself may be a negative regulator of IgG antibodies or it may indirectly regulate cytokines, which drive IgG antibody responses. IgG is composed of four subtypes. Since different IgG subclass antibodies have been reported to be differentially regulated by different cytokines in both the murine and the human systems (reviewed in ref. 6), we next examined if the relationship between IFN-γ and IgG antibodies was general to all IgG subtypes or selective with individual IgG subclasses.
Figure 2.
Correlation of IgG antibody responses to MLSON and IFN-γ responses secreted in response to MLSON (10 μg/ml) in leprosy patients and exposed healthy household contacts. Results are expressed as optical density units [OD×dilutions] obtained in the linear range of the calibration curve generated using a serum pool with high titres of IgG antibodies. IFN-γ responses are given as pg/ml. Each symbol represents an individual donor. Paired comparisons using the two-tailed Fisher exact test were carried out to determine the significance of correlation. The r-, P- and n-values are indicated.
Relationship of T-cell and macrophage cytokine responses in leprosy patients and M. leprae-exposed individuals
The significance of the relationship between T-cell cytokines (IFN-γ, IL-2, IL-5 and IL-10) and macrophage cytokines (IL-6, TNF-α and GM-CSF) was assessed with two immunoglobulin isotypes (G and E) and four IgG (G1–G4) subclasses. Paired comparisons using the two-tailed Fisher exact test was carried out to assess the significance of relationship between various cytokines and circulating antigen-specific antibodies (Table 1). All statistically significant values (P-values) are underlined.
Table 1.
Relationship between MLSON-specific antibody isotypes and secreted cytokines from MLSON-stimulated PBMCs
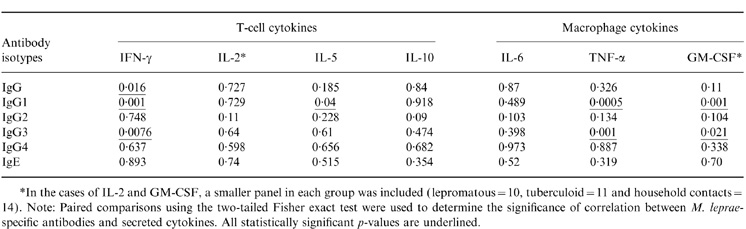 |
Among the T-cell cytokines only IFN-γ showed a highly significant negative correlation with IgG1 (r=−0·402; P<0·001) and IgG3 antibodies (r=−0·35; P=0·007). Thus the negative correlation of IgG with IFN-γ was mainly contributed by IgG1 and IgG3 antibodies. IL-5 showed some relationship with IgG1 responses (P=0·04) but this was much weaker than what was observed with IFN-γ. Also, these results are less convincing since only low levels of IL-5 were detected across the spectrum (<15 pg/ml) in response to MLSON. Since IL-5 is also considered a surrogate marker for Th2 responses, these results suggest low to absent Th2 responses. The low levels of IL-5 were not due to technical problems as adequate IL-5 responses (mean=315 pg/ml; n=58) were detected in response to the mitogen phytohaemagglutinin (data not shown) in the same group. IL-10 is secreted by both T cells and macrophages, but we have included IL-10 with T-cell cytokines as we had collected the supernatants at day 5 for IL-10, as we did for other T-cell cytokines. IL-10, which is reported to act as a switch factor for IgG3 and IgG1 responses11 also did not show any significant relationship with any of the circulating antibody isotypes or subclasses.
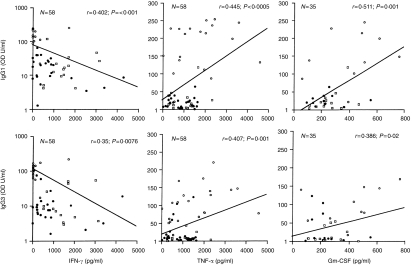
Among the macrophage cytokines IL-6, which is a known activator of B cells, did not show a relationship with MLSON-specific antibodies, suggesting that it may require other costimulatory signals for driving antigen-specific antibody responses. Surprisingly, a highly significant positive correlation was observed between the macrophage cytokines TNF-α and IgG1 and IgG3 antibodies (r=0·445; P=0·0005 and r=0·407; P=0·001), and GM-CSF and IgG1/IgG3 antibodies (r=0·511; P=0·001 and r=0·386; P=0·021), respectively. The significant relationships between cytokines and antibodies are further elaborated in Fig. 3, which depicts the regression line, r-, P- and n-values. While it was not surprising to observe a negative correlation between IFN-γ and IgG subtypes, it was surprising to find a positive relationship between TNF-α and GM-CSF with IgG subtypes IgG1 and IgG3 antibodies since these two cytokines have no reported role in B-cell activation.
Figure 3.
Correlation of IgG1 and IgG3 subclass antibody responses to MLSON and IFN-γ, TNF-α and GM-CSF secreted in response to MLSON (10 μg/ml). Leprosy patients and exposed household contacts were included in the analysis. For IFN-γ and TNF-α the group numbers are the same as in Fig. 1. In the case of GM-CSF a smaller panel was included (L=10, T=11 and HC=14) but all groups were represented. The symbols used for individual groups and the statistical analysis are the same as in Fig. 1.
DISCUSSION
Although IgG1 and IgG3 antibodies are clearly associated with progressive disease in leprosy,7 their role in disease pathogenesis remains to be elucidated. IgG subclasses in both the murine and human systems have been reported to be differentially regulated by cytokines, which act as switch and growth factors for B cells at different stages of maturation.6 Recently, Fujeida et al.17 have reported that CD1-restricted double-negative (CD4− CD8−) T cells in the presence of a characteristic lipoglycan (lipoarabinomannan or LAM) expressed on the surface of mycobacteria drives the response of tonsillar B cells towards IgG1 and IgG3 antibody production. These molecules (CD1) are present on the surface of antigen-presenting cells and have been recently shown to recognize selectively lipid and sugar moieties, which they present to other T and B cells.18,19 CD1 T cells have been isolated from leprosy lesions, where they are over-represented19 probably because of the high concentrations of LAM due to the high bacterial load. If CD1 cells are driving the antibody response of IgG1 and IgG3 antibodies this may also explain the high concentrations of IgG1 and IgG3 antibodies in mycobacterial diseases, particularly in leprosy where these antibodies are positively correlated with the bacterial load.7 This study now addresses the important issue of IgG subclasses and its relationship to various cytokines in leprosy. Since antibody profile can change with even short-term chemotherapy, we were careful to include only patients pretreatment in the study. We also included household contacts with recent exposure to M. leprae to complete the spectrum of infection with M. leprae both pre- and post-disease establishment.
In the murine system the pro-inflammatory complement-fixing antibodies IgG2a and IgG1 (homologous to human IgG1 and IgG3 antibodies) have been shown to be up-regulated by IFN-γ,12 while in the human system, IFN-γ has been shown to be a potent inhibitor of B-cell responses.20 Our results are in keeping with the latter finding and may explain the inverse relationship observed between IFN-γ responses and IgG antibodies across the disease spectrum. Among the four IgG subclasses the main contributors to this negative association were IgG1 and IgG3 antibodies. Thus it would seem that IFN-γ may be differentially regulating different IgG subclasses, as has been reported by other investigators.21 However, the mechanism by which it acts as a negative regulator of IgG antibody responses remains to be elucidated.
We found no correlation between IgG1 and IgG3 antibodies with IL-6, which has been reported to be a key proliferation and terminal differentiating factor of B cells.22 Similarly we did not find any relationship of IL-6 with polyclonal IgG antibody responses either (data not shown). Surprisingly, we also did not find any relationship with IL-10, which has been reported to be a switch factor for IgG3 and IgG1 antibodies.11 Thus, other costimulatory signals, such as CD40 cross-linking, may be the determining factor in IgG subclass antibody switching in leprosy.23
The most interesting finding was the positive association of macrophage cytokines, TNF-α and GM-CSF with IgG1 and IgG3 antibodies. Neither of these cytokines have a direct role in B-cell activation. Since macrophages possess high-affinity receptors for IgG1 and IgG3 antibodies,24 uptake of antigen via the Fc receptors rather than the mannose receptor may modulate cytokine expression by macrophages. This would be an extremely important issue to address since TNF-α is the major determinant of pathogenesis and disease severity in mycobacterial diseases,25,26 as well as leishmaniasis.27 Human mononuclear cells and alveolar macrophages produce large quantities of TNF-α in response M. tuberculosis28 and mycobacterial components such as LAM and other low molecular weight proteins.29,30 We have preliminary evidence which suggests that TNF-α secretion in a purified protein derivative (PPD) stimulated adherent cell population is up-regulated in the presence of PPD-specific IgG1 and IgG3 antibodies, while selective absorption of these antibodies removes the enhancing activity (manuscript in preparation). These results for the first time provide a tangible link between IgG1 and IgG3 antibodies and chronic stimulation of TNF-α in mycobacterial diseases. It is therefore critical to understand the regulation of both IgG1 and IgG3 antibodies and their role in modulating macrophage function.
Acknowledgments
The authors would like to thank The Marie Adelaide Leprosy Centre, Karachi, Pakistan for patient material; Dr Sebastian Lucas, University College School of Medicine, London, UK (currently at St. Thomas Hospital) for providing histopathology on all leprosy patients; and Miss Regina Paul and Mr Akbar Andani for secretarial help. Excellent technical assistance was provided by Miss Nabila Abrar. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
References
- 1.Bullock WE, Watson S, Nelson KE, Schauf V, Makonkawkeyoon S, Jacobson RR. Aberrent immunoregulatory control of B lymphocyte function in lepromatous leprosy. Clin Exp Immunol. 1982;49:105. [PMC free article] [PubMed] [Google Scholar]
- 2.Myrvang B, Godal T, Ridley DS, Froland SS, Song YK. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and hisopathological spectrum of leprosy. Clin Exp Immunol. 1973;14:54. [PMC free article] [PubMed] [Google Scholar]
- 3.Melsom R, Harboe M, Myrvang B, Godal T, Belehu A. Immunoglobulin class specific antibodies to M. leprae in leprosy patients, including the indeterminate groups and healthy contacts as a step in development of methods for serodiagnosis of leprosy. Clin Exp Immunol. 1982;47:225. [PMC free article] [PubMed] [Google Scholar]
- 4.Mutis T, Kraakman EM, Cornelisse YE, et al. Analysis of cytokine production by Mycobacterium-reactive T cells. J Immunol. 1993;150:4641. [PubMed] [Google Scholar]
- 5.Dockrell HM, Young SK, Britton K, et al. Induction of Th1 cytokine responses by mycobacterial antigens in leprosy. Infect Immun. 1996;64:4385. doi: 10.1128/iai.64.10.4385-4389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stavnezer J. Antibody class switching. Adv Immun. 1996;61:79. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- 7.Hussain R, Kifayet A, Chaing TJ. IgG1 and IgG3 are the markers of progressive disease in leprosy. Infect Immun. 1995;63:410. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferis R. Structure-function relationship of IgG subclasses. In: Shakib F, editor. The Human IgG Subclasses Molecular Analysis of Structure, Function and Regulation. Derby: Pergamon Press; 1990. p. 93. [Google Scholar]
- 9.Kifayet A, Hussain R. Selective decrease of M. leprae specific IgG1 and IgG3 antibodies in leprosy patients associated with ENL. Int J Lep. 1996;64:105. [PubMed] [Google Scholar]
- 10.Kifayet A, Shahid F, Lucas SB, Hussain R. Upregulation of polyclonal IgG1 subclass antibody synthesis is associated with ENL reactions in leprosy. Clin Exp Immunol. 1996;106:447. doi: 10.1046/j.1365-2249.1996.d01-860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujeida S, Saxon A, Zhang KE. Direct evidence that γ1 and γ3 switching in human B cells is interleukin 10 dependent. Mol Immunol. 1996;33:1335. doi: 10.1016/s0161-5890(96)00092-2. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-γ regulates the isotypes of Ig secreted in vivo humoral immune responses. J Immunol. 1988;140:1022. [PubMed] [Google Scholar]
- 13.Hussain R, Jamil S, Kifayet A, et al. Quantitation of IgM antibodies to the M. leprae synthetic dissacharide can predict early bacterial multiplication in leprosy. Int J Lep. 1990;50:491. [PubMed] [Google Scholar]
- 14.Hussain R, Poindexter RW, Wistar R, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. I. Development of two site immuno enzymometric assays for total IgG subclass determinations. J Immunol Meth. 1986;93:89. doi: 10.1016/0022-1759(86)90437-0. [DOI] [PubMed] [Google Scholar]
- 15.Hussain R, Poindexter RW, Reimer CB. Use of monoclonal antibodies to quantify subclasses of human IgG. II. Enzyme immunoassay to define filaria specific IgG subclass antibodies. J Immunol Meth. 1986;94:73. doi: 10.1016/0022-1759(86)90217-6. [DOI] [PubMed] [Google Scholar]
- 16.Hussain R, Hamilton RG, Adkinson NF, Kumaraswani R, Ottesen EA. The IgE responses in human filariasis. I. Quantitation of filaria specific IgE. J Immunol. 1981;127:1723. [PubMed] [Google Scholar]
- 17.Fujieda S, Sieling PA, Modlin RL, Saxon A. CD-1 restricted T-cells influence IgG subclass and IgE production. J Allerg Clin Immunol. 1998;101:545. doi: 10.1016/s0091-6749(98)70362-8. [DOI] [PubMed] [Google Scholar]
- 18.Maher JK, Kroneneberg M. The role of CD1 molecules in immune responses to infection. Curr Opin Immunol. 1997;9:456. doi: 10.1016/s0952-7915(97)80095-7. [DOI] [PubMed] [Google Scholar]
- 19.Sieling PA, Chatterjee D, Parcel SA, et al. CD1-restricted T cd l recognition of microbial lipoglycan antigens. Science. 1995;269:227. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds DS, Boom WH, Abbas AK. Inhibition of B lymphocyte activation by interferon-γ. J Immunol. 1987;139:767. [PubMed] [Google Scholar]
- 21.King CL, Nutman TB. IgE and IgG subclass regulation by IL4 and IFN gamma in human helminth infections. J Immunol. 1993;15:458. [PubMed] [Google Scholar]
- 22.Lue C, Kiyono H, McGhee JR, et al. Recombinant human interleukin 6 (rhIL-6) promotes the terminal differentiation of in vivo-activated human B cells into antibody-secreting cells. Cell Immunol. 1991;132:423. doi: 10.1016/0008-8749(91)90039-e. [DOI] [PubMed] [Google Scholar]
- 23.Burdin N, Van Kooten C, Galibert L, et al. Endogenous IL6 and IL10 contribute to the differentiation of CD-40 activated human B lymphocytes. J Immunol. 1995;154:2533. [PubMed] [Google Scholar]
- 24.Anderson CL, Abraham GN. Characterization of the Fc for IgG on a human macrophage cell line, U937. J Immunol. 1980;125:2735. [PubMed] [Google Scholar]
- 25.Santos DO, Suffys PN, Bonifacio K, Marques MA, Sarno EN. In vitro tumor necrosis factor production by mononuclear cells from lepromatous leprosy patients and from patients with erythema nodosum leprosum. Clin Immunol Immunopathol. 1993;67:199. doi: 10.1006/clin.1993.1065. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor in patients with leprosy. Infect Immun. 1992;60:1441. doi: 10.1128/iai.60.4.1441-1446.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisa P, Menistu G, Soder O, Ottenhoff T, Hansson M, Kiessling R. Serum tumor necrosis factor levels and disease dissemination in leprosy and leishmaniasis. J Infect Dis. 1990;161:988. doi: 10.1093/infdis/161.5.988. [DOI] [PubMed] [Google Scholar]
- 28.Valowe SE, Rich EA, Wallis RS, Ellner JJ. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988;56:3313. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes PF, Chatterjee D, Abrams JS, Band H, Rea TH, Modlin RL. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992b;149:541. [PubMed] [Google Scholar]
- 30.Wallis RS, Amir-Tahmasseb M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte Western blot. Proc Nat Acad Sci USA. 1990;87:3348. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]