Abstract
We have studied the appearance and phenotype of recent thymic emigrants in blood, spleen and lymph nodes (LN) of neonatal lambs. Using in situ labelling of thymocytes with fluoroscein isothiocyanate (FITC), we examined the expression of the LN homing receptor l‐selectin on αβ and γδ subsets of recent thymic emigrants 24 hr after labelling. There were marked differences in the proportions of CD4+, CD8+ and γδ T‐cell receptor (TCR+) cells exported from the thymus to spleen compared to lymph nodes. Spleen was enriched in CD8+ and γδ TCR+ emigrants while LN were enriched in CD4+ emigrants. There were also marked differences in the expression of l‐selectin by emigrants homing to spleen compared with those homing to lymph nodes. While the majority of thymic emigrants in LN expressed l‐selectin, considerably fewer emigrants in spleen were l‐selectin+. The presence of large numbers of CD8+ l‐selectin– and γδ TCR+ l‐selectin– thymic emigrants homing to spleen raises the possibility that unique homing receptor specificities underpin the migration of T cells to spleen as distinct from lymph nodes.
Introduction
The immune system relies on the export of T cells from the thymus to create a pool of naive T cells with a diverse repertoire for antigen.1,2 Cells selected from this pool of naive T cells after interaction with cognate antigen give rise to memory cells which provide protection against subsequent exposure to infectious agents. Memory T cells are l‐selectin– and are thought to show different tissue‐specific homing patterns from naive T cells whereby they preferentially target peripheral tissues.3,4 In contrast to memory T cells, naive T cells express l‐selectin and are said to selectively target high endothelial venules (HEV) and lymph nodes (LN).3,4l‐selectin is the key homing receptor mediating lymphocyte entry into LN, as shown by studies using l‐selectin knock‐out mice, where T and B lymphocyte entry into peripheral lymph nodes (PLN) and mesenteric lymph nodes (MLN) is reduced by up to 98%.5 In order to understand better the role of antigen in generating tissue‐selective homing of subsets of T cells, it is important to know the homing properties of T cells immediately after they are exported from the thymus, so that any changes which may occur in their homing specificities after export as a consequence of activation by antigen can be determined.
In this study we have used the technique of in situ labelling of the thymus with fluorescein isothiocyanate (FITC), which has been used extensively in many species to study thymic export,6–12 in order to examine the tissue localization of thymic emigrants. We report the presence of two main streams of thymic emigrants with different homing specificities. A population of l‐selectin+, predominantly CD4+ emigrants homing to LN, and a population of predominantly CD8+l‐selectin– emigrants homing to spleen.
Materials and methods
Animals
Merino lambs of 4–12 weeks of age were obtained by a controlled mating programme and born on site in our animal house facility.
In situ thymic labelling with FITC
The FITC labelling of the cervical thymus was performed as described previously in mice7 and sheep.12 Briefly, the thymus was exposed under anaesthesia by a midline incision in the neck of the lamb and blunt dissection of the overlying tissue. An aqueous solution of FITC (approximately 500 µg/ml in phosphate‐buffered saline (PBS)) was prepared as described by Scollay,7 and approximately 15 ml was injected directly into the thymus at multiple sites. The incision was then closed with interrupted silk sutures.
Cell suspensions, peripheral blood lymphocytes (PBL) isolation and cell counting
Cell suspensions were prepared by teasing the tissues through a wire mesh into PBS containing 2% bovine serum albumin (BSA), 0·4% ethylenediamine tetra‐acetic acid (EDTA) and 0·1% sodium azide, then washed three times in the same medium (PBS/BSA). Blood was collected from the jugular vein in EDTA (10 mg/ml blood) and red cells were lysed using Tris‐buffered ammonium chloride,13 then washed three times in PBS/BSA.
Cell concentrations were determined using a Coulter Counter (Industrial D, Coulter, Dunstable, UK). Total white cell counts were determined using a haemocytometer, and differential white cell counts were made on Giemsa‐stained smears.
Monoclonal antibodies
The monoclonal antibodies (mAb) recognizing the sheep lymphocyte surface antigens CD5 (25‐91),14 CD4 (44‐38 and 44‐97)15 and CD8 (38‐65)15 were obtained from Dr M. Brandon (The University of Melbourne, Victoria, Australia). Those against the γδ T‐cell receptor (γδ TCR, 86D),16 surface immunoglobulin+ (B) lymphocytes17 and the peripheral LN homing receptor, l‐selectin (Du1‐29),18 were obtained from Dr C. Mackay (Leukosite Inc., Boston, MA). The mAb were used as ascites at a previously determined optimal concentration of 1/100–1/10 000 or as 1/2 diluted hybridoma supernatant. Isotype‐matched antibodies against non‐lymphocyte antigens were used as controls for non‐specific binding.
Immunofluorescent staining and flow cytometry
The phenotypes of FITC‐labelled and mature cells were determined using single and two‐colour immunofluorescence staining. All steps were performed at 4°, and all incubations were for 30 min. All washes were performed three times using PBS/BSA as the wash buffer. For single colour stains, aliquots containing 8 × 106 cells were incubated with 50 µl mAb, washed, then resuspended in 50 µl phycoerythrin (PE)‐conjugated sheep anti‐mouse immunoglobulin F(ab′)2 (AMRAD Biotech, Boronia, Victoria, Australia). Cells were then washed and fixed in 3% formaldehyde. For two‐colour staining, aliquots containing 8 × 106 cells were incubated with 50 µl anti‐l‐selectin mAb, washed, then resuspended in 50 µl PE‐conjugated sheep anti‐mouse Ig F(ab′)2. Cells were then incubated with a 10% solution of normal mouse serum in order to saturate free binding sites on the second antibody. Finally, cells were washed, incubated with 50 µl biotinylated anti‐CD4, anti‐CD8 or anti‐γδ mAb followed by 50 µl TriColor (Caltag Laboratories, San Fransisco, CA), washed and then fixed. All samples were analysed within 5 days of fixation on a fluorescence‐activated cell sorter (FACScan flow cytometer, using either Consort 30 or FACScan software; Becton Dickinson FACS Systems, Sunnyvale, CA). Dead cells, granulocytes and debris were excluded on the basis of forward angle and 90° light scatter. The percentages of FITC‐labelled cells in unstained samples were determined from five collections of 5 × 104 cells for each time point. For each mAb 2–5 × 103 cells were collected to determine the phenotypes of the mature cells, and separate collections of 1–3 × 103 FITC‐labelled cells were made triggering on fluorescence‐1 to exclude unlabelled cells.
Statistical analysis
The percentages or numbers of each subset were compared between lymphocyte sources using a one‐way analysis of variance. Those showing variation were then compared using an unpaired Student’s t‐test.19 A value of P < 0·05 was considered significant.
Experimental protocol
Twenty‐four hours after in situ labelling with FITC, animals were killed and tissue samples taken for phenotypic analysis of thymic emigrants (FITC+) and mature T cells (FITC–). Blood and spleen tissue samples were taken, along with prescapular, prefemoral, popliteal, caudal mediastinal (CMS) and mesenteric LN. Lungs, liver, bone marrow and gut contained very few thymic emigrants, and the overwhelming majority of emigrants were found in blood, spleen and LN, as reported previously.7
The percentage of thymocytes labelled with FITC was determined by FACs analysis and the number of emigrants in the periphery corrected for 100% thymus labelling. The total number of emigrants in blood at 24 hr was estimated using the weight of the animal to calculate total blood volume (74·0 ml/kg20).
The total number of emigrants in peripheral tissues was calculated from the weight of the tissue and the number of lymphocytes recovered per gram of tissue, after allowing for cells lost during the preparation of cell suspensions.7 The number of emigrants was considered to be equal to those in the blood and spleen plus twice those in the pool of LN studied, as previously described.21 The daily export rate of T cells was expressed as the percentage of thymocytes exported per day.
Results
CD8+ thymic emigrants home predominantly to spleen, whereas CD4+ emigrants home predominantly to LN
Table 1 shows the average daily thymic export rate was 0·9% thymocytes/day in young lambs, which is similar to that previously reported in sheep,6,22 mice,7 rats,8 chickens9 and pigs.11 The majority of thymic emigrants were found in the spleen (72%), while blood contained 10%, and LN 18% of thymic emigrants recovered 24 hr after in situ labelling with FITC.
Table 1.
Daily export rate of thymic emigrants
| Tissue | Number of thymic emigrants exported/day (×10−8)* | Total thymic emigrants exported/day† (%) |
|---|---|---|
| Blood | 1·61 ± 0·31 | 10 |
| Spleen | 12·03 ± 0·84 | 72 |
| LN | 2·99 ± 0·26 | 18 |
| Total | 16·64 ± 0·85 | 100 |
| Total number ofthymocytes | 1974·00 ± 2·06 | |
| % thymocytes exported/day | 0·90 ± 0·11 |
The distribution of thymic emigrants in blood, spleen and pooled peripheral and mesenteric LN 24 hr after in situ labelling with FITC.
Mean ± SEM of six animals.
The number of thymic emigrants found in individual tissues expressed as a percentage of the total number of thymic emigrants recovered 24 hr after in situ labelling with FITC.
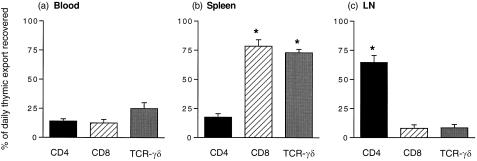
When the distribution of emigrant subsets was examined in blood, spleen and LN, marked differences in the homing of thymic emigrants to each of these tissues was found. Figure 1 shows that CD8+ thymic emigrants in the spleen accounted for 78% of the daily thymic export of CD8+ T cells. Spleen also captured the majority (73%) of γδ TCR+ thymic emigrants. In contrast, the majority of CD4+ emigrants (64%) was recovered in the LN.
Figure 1.
Percentage of total daily thymic export of CD4+, CD8+, and γδ TCR+ thymic emigrants recovered in blood, spleen and LN 24 hr after in situ labelling with FITC. The data represents the mean ± SEM of six animals. *Significant difference from the same subset in other tissues.
A comparison of the CD4 : CD8 ratios of both emigrants and mature T cells in blood, spleen and LN suggested that CD8+ emigrants were being lost from the spleen where thymic emigrants were found in a CD4 : CD8 ratio of 1 : 15 as opposed to 1 : 5 in the mature T‐cell pool (Table 2). If no selective proliferation of T‐cell subsets is occurring, these results raise the possibility that selective loss of CD8+ emigrants from the spleen was occurring, either through emigration to other tissues (for which we could find no evidence), or through cell death of CD8+ emigrants in situ in the spleen. The situation was different in LN where the CD4 : CD8 ratio of 3 : 1 remained stable in both the emigrant and mature pool suggesting that CD8+ emigrants homing to LN were not being depleted in the same way as those found in spleen (Table 2).
Table 2.
The CD4 : CD8 ratio of thymic emigrants and mature T cells in tissues 24 hr after in situ labelling with FITC*
| Thymic emigrants CD4 : CD8 ratio | Mature T cells CD4 : CD8 ratio | |
|---|---|---|
| Blood | 1 : 3 | 1 : 1 |
| Spleen | 1 : 15 | 1 : 5 |
| LN | 3 : 1 | 3 : 1 |
The data represents the mean of six animals.
l‐selectin expression on thymic emigrants defines two distinct tissue‐specific migration patterns
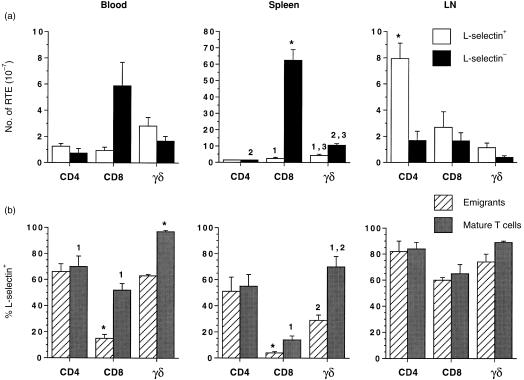
Over 95% of CD8+ thymic emigrants in spleen were l‐selectin– and overall CD8+ l‐selectin– emigrants constituted 75% of the total number of emigrants which homed to spleen (Fig. 2a). This was in marked contrast to CD4+ emigrants that homed preferentially to LN where over 80% of CD4+ emigrants were l‐selectin+ and amounted to 51% of all emigrants localizing in LN (Fig. 2a). The majority of γδ TCR+ emigrants recovered in the spleen 24 hr after in situ thymus labelling were also l‐selectin–, in contrast to blood and LN where there were more l‐selectin+ γδ TCR+ emigrants. Although the majority of mature CD8+ T cells in the spleen were l‐selectin–, nonetheless significantly more mature CD8+ T cells than thymic emigrants in blood and spleen were l‐selectin+ (Fig. 2b), suggesting that either there had been selective loss of CD8+ l‐selectin– T cells in blood and spleen, through cell death or migration to other tissues, or that post‐thymic upregulation of l‐selectin expression was occurring on these subsets.
Figure 2.
(a) The number of l‐selectin+ and l‐selectin– CD4+, CD8+ and γδ TCR+ thymic emigrants in blood, spleen and peripheral LN 24 hr after in situ labelling with FITC. The data represents the mean ± SEM of four animals. (b) The percentage distribution of l‐selectin+ emigrant and mature CD4+, CD8+ and γδ TCR+ T cells in blood, spleen and LN 24 hr after in situ labelling with FITC. The data represents the mean ± SEM of four animals. *Significant difference from all other subsets within the tissue. 1,2,3, significant difference from other subset with the same number within the tissue.
Discussion
The experiments reported here suggest that the thymus exports two distinct streams of thymic emigrants with different homing specificities. A population of CD8+ l‐selectin– emigrants comprised 75% of all thymic emigrants localizing in spleen 24 hr after in situ thymus labelling with FITC. In contrast, 51% of all thymic emigrants localizing in LN were CD4+ l‐selectin+. Smaller populations of CD8+ and γδ TCR+ thymic emigrants homing to LN were also predominantly l‐selectin+. l‐selectin expression on thymic emigrants thus appears to define two distinct pathways of migration, one homing to spleen and the other to LN.
The thymus is responsible for the development of the peripheral T‐cell pool, which includes a population of naı¨ve T cells recirculating between blood, LN and spleen.23–25 Although many studies have reported altered migration of T cells after interaction with antigen, there have been relatively few reports on the role of the thymus in the induction of tissue‐selective homing of antigen inexperienced T cells. It is known that the LN homing receptor, l‐selectin, is expressed on medullary thymocytes and some, but not all thymic emigrants,12,26 and that the majority of thymic emigrants migrate to spleen and LN.7 It is not known, however, if thymic emigrants form a relatively homogeneous pool of T cells randomly exchanging between blood, LN and spleen, or whether separate populations of thymic emigrants exist, which home specifically to spleen and LN as a result of distinct tissue‐homing specificities that are conferred during thymic development.
The experiments reported here raise the possibility that thymic emigrants do not form homogeneous populations of T cells freely exchanging between LN and spleen, but rather consist of cells specifically targeting spleen or LN. While post‐thymic down‐regulation of l‐selectin on thymic emigrants homing to spleen cannot be excluded in our experiments, analysis of l‐selectin+ emigrants in blood at 3 hr suggested that the number of l‐selectin+ emigrants exported was similar to the number recovered (data not shown). While there is abundant evidence that the expression of l‐selectin on T cells is mandatory for homing to LN,5 and the majority of LN homing emigrants in this report were l‐selectin+, the question of l‐selectin– T cells homing to the spleen is less clear. The actual mechanisms of lymphocyte entry into the spleen have yet to be elucidated; however, a number of reports indicate that entry of T cells into spleen is mediated by l‐selectin‐independent mechanisms. Weston and Parish27 demonstrated in mice that blocking of splenic sinusoidal cells by mannan stopped splenic entry of lymphocytes but did not affect entry into LN.
Although our experiments cannot exclude the possibility that lacking l‐selectin, thymic emigrants accumulate by default in the spleen, we currently favour the notion that thymic emigrants are specifically homing to the spleen. Several recent reports on recirculating and non‐recirculating lymphocyte subpopulations in the sheep support the concept of tissue‐specific migration of lymphocytes to spleen. Two distinct populations of B cells have also been described in sheep, one of which is CD21– and l‐selectin– and which circulates between blood and spleen and does not enter LN, while the second, CD21+ l‐selectin+ population recirculates via HEV in LN into lymph.28 Gupta et al.29 also described these two B‐cell subsets and found that the non‐recirculating l‐selectin– population of B cells in sheep is CD11b+ immunoglobulin Mhi (IgMhi) and populates the splenic marginal zone and blood, while the second l‐selectin+ recirculating B‐cell population is CD11b+ IgMlo. Andrade et al.30 also identified a population of γδ TCR+ cells in blood that did not recirculate through efferent LN. A large population of l‐selectin– cells has also been described in human spleen.31
A comparison of the CD4 : CD8 ratios of mature T cells versus thymic emigrants in the spleen raised the possibility that CD8+ l‐selectin– emigrants were dying in the spleen. The observation that l‐selectin expression was more prevalent on mature T cells than emigrants in spleen could also be explained by high cell death rates among splenic CD8+ l‐selectin– emigrant populations, although post‐thymic maturation ofl‐selectin expression on thymic emigrants could also be a contributing factor. We are currently examining the lifespan of thymic emigrants using long‐term lymphocyte tracking dyes.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. E.A. Washington is a NH & MRC Senior Research Officer. J.E. Holder is the holder of a Dora Lush (NH & MRC) Scholarship.
References
- 1.Tanchot C, Rosado MM, Agenes F, Freitas AA, Rocha B. Lymphocyte homeostasis. Semin Immunol. 1997;9:331. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 2.Tanchot C, Rocha B. The organization of mature T‐cell pools. Immunol Today. 1998;19:575. doi: 10.1016/s0167-5699(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 3.Picker LJ, Treer JR, Ferguson‐Darnell B, Collins PA, Buck D, Terstappen LW.M.M. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte‐associated antigen, a tissue‐selective homing receptor for skin‐homing T cells. J Immunol. 1993;150:1122. [PubMed] [Google Scholar]
- 4.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 5.Tang MLK, Steeber DA, Zhang X‐q, Tedder TF. Intrinsic differences in l‐selectin expression levels affect T and B lymphocyte subset‐specific recirculation pathways. J Immunol. 1998;160:5113. [PubMed] [Google Scholar]
- 6.Miyasaka M, Pabst R, Dudler L, Cooper M, Yamaguchi K. Characterization of lymphatic and venous emigrants from the thymus. Thymus. 1990;16:29. [PubMed] [Google Scholar]
- 7.Scollay R, Butcher E, Weissman I. Thymus cell migration: quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 8.Hosseinzadeh H, Goldschneider I. Recent thymic emigrants in the rat express a unique antigenic phenotype and undergo post‐thymic maturation in peripheral lymphoid tissues. J Immunol. 1993;150:1670. [PubMed] [Google Scholar]
- 9.Katevuo K, Vainio O. Thymocyte emigration in the chicken: an over‐representation of CD4+ cells over CD8+ in the periphery. Immunology. 1996;89:419. doi: 10.1046/j.1365-2567.1996.d01-764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunon D, Courtois D, Vainio O, et al. Ontogeny of the immune system: γ/δ and α/β T cells migrate from the thymus to the periphery in alternating waves. J Exp Med. 1997;7:977. doi: 10.1084/jem.186.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binns RMP, Pabst R, Licence ST. Subpopulations of T lymphocytes emigrating in venous blood draining pig thymus labelled in vivo with fluorochrome. Immunology. 1988;63:261. [PMC free article] [PubMed] [Google Scholar]
- 12.Witherden DA, Abernethy NJ, Kimpton WG, Cahill RNP. Changes in thymic export of l‐selectin+γδ and αβ T cells during fetal and postnatal development. Eur J Immunol. 1994;24:1234. doi: 10.1002/eji.1830240535. [DOI] [PubMed] [Google Scholar]
- 13.Mackay CR, Kimpton WG, Brandon MR, Cahill RNP. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J Exp Med. 1988;167:1755. doi: 10.1084/jem.167.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackay CR, Maddox JF, Gogolin‐Ewens KJ, Brandon MR. Characterization of two sheep lymphocyte differentiation antigens, SBU‐T1 and SBU‐T6. Immunology. 1985;55:729. [PMC free article] [PubMed] [Google Scholar]
- 15.Maddox JF, Mackay CR, Brandon MR. Surface antigens, SBU‐T4 and SBU‐T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985;55:739. [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay CR, Beya M‐f, Matzinger P. γ/δ T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989;19:1477. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- 17.Beh KJ. Monoclonal antibodies against sheep immunoglobulin light chain, IgM and IgA. Vet Immunol Immunopathol. 1988;18:19. doi: 10.1016/0165-2427(88)90033-5. [DOI] [PubMed] [Google Scholar]
- 18.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, Hein WR. Tissue‐specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992;22:887. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- 19.Glantz SA. Primer of Biostatistics. 2. New York: McGraw‐Hill; 1987. [Google Scholar]
- 20.Moritz KM, Owens PC, Wintour EM. Changes in blood and red cell volume in the neonatal lamb and the effect of insulin‐like growth factor I. Clin Exp Pharmacol Physiol. 1996;23:134. doi: 10.1111/j.1440-1681.1996.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 22.Witherden DA, Kimpton WG, Abernethy NJ, Cahill RNP. Changes in thymic export of γ/δ and αβ T cells during fetal and postnatal development. Eur J Immunol. 1994;24:2329. doi: 10.1002/eji.1830241011. [DOI] [PubMed] [Google Scholar]
- 23.Weissman IL. Thymus cell migration. J Exp Med. 1967;126:291. doi: 10.1084/jem.126.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JF.A.P. Immunological function of the thymus. Lancet. 1961;ii:748. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 25.Miller JF.A.P. The discovery of the immunological function of the thymus. Immunol Today. 1991;12:42. doi: 10.1016/0167-5699(91)90111-6. [DOI] [PubMed] [Google Scholar]
- 26.Reichert RA, Gallatin WM, Butcher EC, Weissman IL. A homing receptor‐bearing cortical thymocyte subset: implications for thymus cell migration and the nature of cortisone‐resistant thymocytes. Cell. 1984;38:89. doi: 10.1016/0092-8674(84)90529-4. [DOI] [PubMed] [Google Scholar]
- 27.Weston SA, Parish CR. Evidence that mannose recognition by splenic sinusoidal cells plays a role in the splenic entry of lymphocytes. Eur J Immunol. 1992;22:1975. doi: 10.1002/eji.1830220804. [DOI] [PubMed] [Google Scholar]
- 28.Young AJ, Marston WL, Dessing M, Dudler L, Hein WR. Distinct recirculating and non‐recirculating B‐lymphocyte pools in the peripheral blood are defined by coordinated expression of CD21 and l‐selectin. Blood. 1997;90:4865. [PubMed] [Google Scholar]
- 29.Gupta VP, McConnell I, Dalziel RG, Hopkins J. Two B cell subpopulations have distinct recirculation characteristics. Eur J Immunol. 1998;28:1597. doi: 10.1002/(SICI)1521-4141(199805)28:05<1597::AID-IMMU1597>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 30.Andrade WN, Johnston MG, Hay JB. The relationship of blood lymphocytes to the recirculating lymphocyte pool. Blood. 1998;91:1653. [PubMed] [Google Scholar]
- 31.Wallace DL, Beverley PCL. Characterization of a novel subset of T cells from human spleen that lacks l‐selectin. Immunology. 1993;78:623. [PMC free article] [PubMed] [Google Scholar]