Abstract
Interactions between B and CD4+ T cells are central to the pathogenesis of retrovirus‐induced murine acquired immune deficiency virus (MAIDS). Prompted by previous work showing that treatment with cytotoxic T lymphocyte antigen 4 immunoglobulin (CTLA4Ig) partly inhibited the disease, we studied the course of infection in mice deficient for CD28–B7 interactions (mCTLA4‐Hγ1 transgenic mice). Despite a relative viral load identical to that of non‐transgenic mice, the transgenic mice did not develop any of the major MAIDS symptoms (i.e. lymphoproliferation and immune anergy). The mCTLA4‐Hγ1 did not however, completely inhibit B‐cell activation as indicated by a slight hypergammaglobulinaemia and microscopic blastic transformation. Absence of MAIDS in transgenic mice was associated with much lower levels of both interleukin‐4 and interferon‐γ transcripts following viral infection. These results support the theory that the CD28/B7 costimulatory pathway is a critical determinant to MAIDS development.
Introduction
Mouse acquired immune deficiency virus (MAIDS) is induced by murine leukaemia viruses (MuLV) present in a virus mixture recovered from a radiation‐induced lymphoma of C57BL/6 mice.1,2 The crucial component of this preparation is a replication‐defective retrovirus, designated BM5def3 or DU5H,4 with a single open reading frame encoding a Pr60gag protein. The syndrome is characterized by a rapid and persistent proliferation of B and CD4+ T cells, hypergammaglobulinaemia, phenotypic abnormalities of lymphocyte subsets, and increasingly severe defects of cellular and humoral immunity.5 Although B cells are the main target for defective viral expression,6 development of the disease is strictly dependent on the presence of functional CD4+ T cells.7 Mechanisms involved in CD4+ T‐cell contribution to MAIDS pathogenesis have been only partly elucidated. Of most importance was the demonstration that chronic T‐cell activation and induction of anergy is major histocompatibility complex (MHC) class II antigen‐dependent.8 More recent research has focused on the costimulatory signalling pathways possibly involved in MAIDS: antibodies against certain adhesion molecules9 or against CD40‐ligand expressed on T cells10,11 were found to inhibit MAIDS development significantly.
Binding of the T‐cell receptor CD28 to its counter‐receptors B7.1 (CD80) and B7.2 (CD86) on antigen‐presenting cells (APC) is now recognized as one of the most potent accessory activating signals to T cells.12,13 The demonstration of an increased expression of B7 molecules on B cells in MAIDS14 prompted us to analyse the effect of a functional blockade of these ligands on MAIDS development. A soluble fusion protein of cytotoxic T lymphocyte antigen 4 (CTLA4) binds to B7 with high avidity and acts as a competitive inhibitor of physiological CD28–B7 interactions.15 In mice inoculated with the MAIDS‐inducing preparation and treated with CTLA4 immunoglobulin (CTLA4Ig) (3 × 50 µg/week), lymphoproliferation progressed at a much slower rate than in untreated mice and the loss of in vitro responsiveness to mitogens was reduced.14 However, the inhibitory effects of CTLA4Ig were circumvented with time, so the importance of CD28/B7 interactions for MAIDS induction was actually difficult to discern in this model. To investigate this question, we analysed the fate of MAIDS virus inoculation in mice transgenic for mCTLA4‐Hγ1 [a fusion protein between mouse CTLA4 and human immunoglobulin G1 (IgG1)]. The generation of transgenic (tg) mice (called mCTLA4‐hγ1 tg hereafter) has been described previously.16 This system allows us to reach and maintain higher serum levels of the transgenic protein than iterative injection of the soluble CTLA4Ig to non‐transgenic mice. Such serum levels are more likely to saturate binding in vivo.
Materials and methods
Mice and virus
The mCTLA4‐Hγ1 transgenic mice, produced by Peter Lane (Basel Institute for Immunology, Basel, Switzerland), were maintained in our facility by breeding transgenic males to C57BL/6 females, under pathogen‐free conditions. Progeny was tested at 5 weeks of age by determining the presence in the serum of the transgenic product, using a competitive inhibition immunoassay for human γ‐heavy chain. Transgenic animals and their control littermates were inoculated three times intraperitoneally (i.p.) at the ages of 6, 7 and 8 weeks with a MAIDS‐inducing viral preparation, obtained as filtered culture medium of Du5H‐transfected SIM.R fibroblasts chronically infected with the replication‐competent RadLV G6T2.17 This preparation was quantified by XC plaque assay18 to contain 1000 plaque‐forming units (PFU) of ecotropic virus/ml. At different time intervals (time 0 = first viral inoculation), mice were killed by CO2 asphyxiation. At autopsy, spleen (SP) and lymph nodes (LN) (axillary, inguinal and mesenteric) were weighed on a precision scale.
Histopathology
Tissue samples were fixed in 4% paraformaldehyde, embedded in glycolmetacrylate (JB Polyscience, Polylab, Antwerp, Belgium); semi‐thin sections (2 µm) were stained with haematoxylin and eosin and histoenzymological staining was performed for acid phosphatases.
Proliferation tests
Single cell suspensions from SP and LN were prepared with a fitting glass homogeneizer and were suspended in RPMI‐1640 complete medium (Gibco, Meselbeke, Belgium). Aliquots containing 2 × 105 cells per 200 ml were cultured in triplicate in 96‐well microtest plates (Nunc, Meselbeke, Belgium) for 72 hr with concanavalin A (Con A) (Boehringer Mannheim, Mannheim, Germany), 5 µg/ml, or lipopolysaccharide (LPS; Difco, Detroit, MI), 10 µg/ml. During the last 4 hr of culture, cells were incubated with 0·4 µCi [3H]thymidine (Dupont®, NEN products, Boston, MA) and collected with a cell harvester (Skatron®, Sterling, VA) onto glass fibre filters. Incorporated precursor was counted in a scintillation analyser (Tri‐Carb®, Packard, Meriden, CT).
Immunoglobulin serum levels
The serum levels of immunoglobulin isotypes were measured by isotype‐specific sandwich enzyme‐linked immunosorbent assay (ELISA). Ninety‐six‐well ELISA plates were coated with antibodies specific for each murine immunoglobulin isotype (rat monoclonal antibodies to murine γ1‐, γ2a‐, γ2b‐, γ3‐ and µ‐heavy chains: LO‐MG1‐13, LO‐MG2a‐7, LO‐MG2b‐2, LO‐MG3‐13 and LO‐MM‐3; Technofarm Biotechnology, Clichy, France) diluted at 5 or 10 µg/ml in phosphate‐buffered saline (PBS)–Tween. Thereafter, serum samples at the concentration of 1/100 (for determination of IgG2b and IgG3 isotype levels) or 1/1000 (for the other isotypes tested) in PBS–Tween were serially diluted and incubated. Horseradish peroxidase (HRPO)‐labelled antibodies to murine heavy chains (LO‐MG1‐2, LO‐MG2a‐3, LO‐MG2b‐1, LO‐MG3‐7 and LO‐MM‐8, Technofarm Biotechnology) were added and incubated. HRPO activity was visualized with o‐phenylenediamine and hydrogen peroxide, with quantification at 490 nm by an ELISA reader (Dynatech Laboratories, Inc., Alexandria, VA). Concentrations of the samples were determined by comparison with standard curves generated with purified isotypes.
Flow cytometry analysis
For staining, 106 cells were incubated with anti‐FcγRII (CD32, Fc block™, Pharmingen, San Diego, CA) to block non‐specific interactions, prior to labelling for 20 min with 50 µl of rat monoclonal antibodies against the following mouse surface antigens: Thy‐1.2 (30‐H12), CD4 (GK 1.5), B220 (RA3‐6B2), all purchased from Pharmingen, and CD8 (53‐6‐72), purified in our laboratory. All incubations were carried out on ice in PBS supplemented with 2% (v/w) bovine serum albumin and 0·1% sodium azide. Antibodies were fluorescein isothiocyanate‐ (FITC), zodophytan phycoerythrin‐ (R‐PE), or biotin‐labelled. When biotinylated antibodies were used, cells were washed twice and counterstained with streptavidin–PE (Becton Dickinson, Erembodegem, Belgium). After additional washes, cells were analysed on a FACStar Plus® cell sorter (Becton Dickinson). Lymphocytes were gated according to forward and side scatter dot plots.
Cytokine gene expression
The RNAse protection assays were performed on 20 µg total RNA extracted from individual spleen samples with the Riboquant Multi probe RNAse protection assay (Pharmingen) according to the manufacturer's instructions. A multiprobe set including specific templates for interleukin‐4 (IL‐4), IL‐5, tumour necrosis factor‐α (TNF‐α), IL‐13, IL‐15, IL‐9, IL‐12, IL‐6, interferon‐γ (IFN‐γ) mRNAs and for the house‐keeping genes L32 and GAPDH was used.
Defective virus expression
Transcripts for Du5H gag protein in spleen cells were detected by reverse transcriptase–polymerase chain reaction (RT‐PCR) technique. RNA samples were prepared following the RNAzol™ B method (Biotecx, Houston, TX) and 2 µg of individual samples were reacted with RT. Primers used for PCR reactions were 5′‐CCTCTTCCTTTATGGACACT‐3′ and 5′‐ATTAGGGGGGGAATAGCTCG‐3′ sequences, corresponding to nucleotides 1282–1301 and 1499–1518, respectively, of the published sequence.19 The DNA of pDu5H was used as a positive control. Quantification of defective gag included normalization to amplification of hypoxanthine phosphoribosyltransferase (HPRT) message. Primers used for HPRT sequence were 5′‐GTTGGATACAGGCCAGACTTTGTTG‐3′ and 5′‐GATTCAACTTGCGCTCATCTTAGGC‐3′.20 Thirty‐cycle amplification PCR was verified to be below saturating conditions.
Results
Twenty‐one mCTLA4‐Hγ1 transgenic mice and 17 non‐transgenic littermates were inoculated with Du5H(G6T2) and assessed for MAIDS symptoms at either 6, 10, or 13 weeks after viral infection; 11 mCTLA4‐Hγ1 transgenic mice and 15 non‐transgenic littermates, sham‐inoculated with sterile PBS, were also analysed as non‐infected controls.
Lymphoproliferation
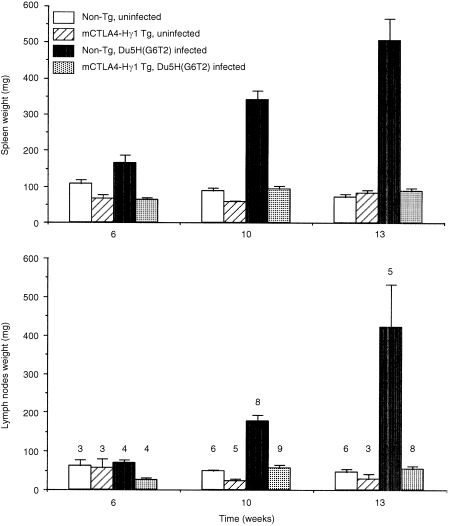
As expected, infected non‐transgenic mice exhibited a progressive increase of spleen (SP) and lymph nodes (LN) weights. Figure 1 represents the means of SP and LN weight recorded from experimental animals examined at the three time‐points after viral or sham‐inoculation. Sham‐inoculation of non‐transgenic mice did not induce any variation in the weight of lymphoid organs. As previously reported by Lane et al. both the SP and LN weight from mCTLA4‐Hγ1 transgenic mice tended to be smaller than those of their non‐transgenic littermates.16 In sharp contrast with non‐transgenic mice, infection of mCTLA4‐Hγ1 transgenics resulted in little or no increase in SP or LN size. Among transgenic animals examined at the 6th week, SP and LN weights from those infected were even lower than those sham‐inoculated. At the 10th and 13th weeks, there was a slight increase in the weights recorded from infected transgenics when compared to uninfected ones; statistically, the difference was significant only for the values at the 10th week. At either the 10th or the 13th week, mean weights of SP and LN of infected transgenics did not significantly differ from those of non‐transgenic uninfected controls.
Figure 1.
The mCTLA4‐Hγ1 transgenic mice do not develop MAIDS‐associated lymphoproliferation. Columns represent means of SP and LN weights ± SEM for each experimental group at 6, 10 and 13 weeks after viral inoculation. The number of mice studied at each time‐point is indicated over the top of the corresponding columns.
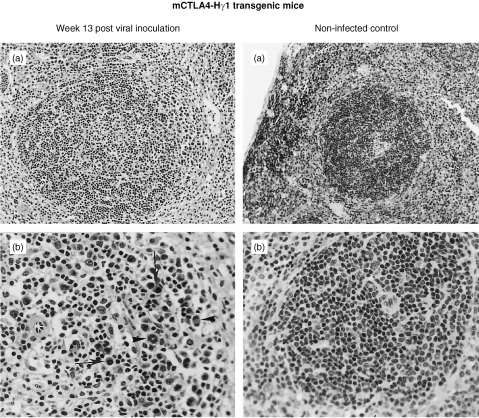
Despite the paucity of macroscopic changes after Du5H(G6T2) infection of mCTLA4‐Hγ1 transgenic mice, histopathological examination of the corresponding spleen specimens revealed significant microscopic changes: there was a discrete expansion and partial blastic transformation of the white pulp, taking place in the periarteriolar lymphoid sheaths (PALS), which filled with blastic cells of the immunoblastic type or with plasmocytoid features. Foci of plasmablasts were also observed in the red pulp (Fig. 2).
Figure 2.
Histological appearance of spleen removed from mCTLA4‐Hγ1 transgenic mice. In the left panel, MAIDS‐infected tg mouse at the 13th week after viral inoculation (haematoxylin and eosin). (a) Concentric enlargement of the periarteriolar lymphoid sheath around the central arteriole (×100) (b) Higher magnification of the same field showing large blastic lymphoid cells of the immunoblastic type or with plasmocytoid features (arrows); note the presence of several mitosis (arrowheads) (×250). By comparison, in the right panel, spleen from a non‐infected transgenic mouse contains small lymphoid nodules (×100) (a), composed mainly of small lymphoid cells (×250) (b).
In vitro proliferative responses to mitogens
Proliferative responses of SP cells from non‐transgenic infected mice were substantially reduced at the 10th week and almost totally abrogated at the 13th week (Table 1). In sham‐inoculated transgenics, responses were in the same range as in non‐transgenic controls. Viral inoculation of mCTLA4‐Hγ1 transgenic mice did not induce any significant variation in their ability to respond to both Con A‐ and LPS‐induced proliferation (Table 1).
Table 1.
Comparison of the proliferative response of splenocytes from uninfected or infected normal and transgenic mice to concanavalin A (Con A) and lipopolysaccharide (LPS)
| mCTLA4‐Hγ1 TG | Infection | n | Unstimulated | Con A | LPS |
|---|---|---|---|---|---|
| 10th week | |||||
| − | − | 4 | 613 ± 213 | 24130 ± 4104 | 29510 ± 6515 |
| − | + | 7 | 431 ± 102 | 7340 ± 1871 | 15852 ± 1865 |
| + | − | 3 | 1395 ± 357 | 26 283 ± 2600 | 26 481 ± 9958 |
| + | + | 4 | 1582 ± 370 | 33 424 ± 2625 | 23 469 ± 2567 |
| 13th week | |||||
| − | − | 2 | 838 ± 244 | 54 025 ± 5291 | 36 408 ± 1783 |
| − | + | 2 | 981 ± 228 | 1949 ± 1089 | 4616 ± 2052 |
| + | − | 1 | 2409 | 75464 | 33818 |
| + | + | 3 | 4301 ± 2000 | 55 347 ± 2416 | 24 520 ± 3022 |
Values (c.p.m.) given in the table represent the arithmetic means of the c.p.m. obtained from each mouse ± SEM; n is the number of mice in each experimental group. For each mouse, c.p.m. values were calculated from triplicate wells (variations between triplicate wells were always less than 10%)
Serum immunoglobulin isotype levels
B cell activation occurring in MAIDS is associated with hypergammaglobulinaemia.2,21 Accordingly, the serum levels of IgG2a, IgG3 and IgM quantified by ELISA, were found to be several fold increased in Du5H(G6T2)‐inoculated non‐transgenic mice (Table 2). IgG2a mostly increased, to 20‐fold the values from controls, whereas the increase in IgG3 and IgM was in the five‐ to 10‐fold range. As previously described,22 the levels of IgG2b and IgG1 were little modified after infection (not shown). Uninfected mCTLA4‐Hγ1 transgenic mice had serum levels of immunoglobulin isotypes slightly lower but in the same range as those from non‐tra0nsgenics. After infection with Du5H(G6T2), transgenic mice developed moderate hypergammaglobulinaemia affecting the same subclasses as those found to be expanded in non‐transgenic infected animals; the total serum concentration of immunoglobulin (G2a + G3 + M) in transgenics was however, only 25% of that of non‐transgenics.
Table 2.
Comparison of the levels of IgG2a, IgG3 and IgM isotypes in sera from uninfected or infected normal and transgenic mice
| mCTLA4‐Hγ1 TG | Infection | n | IgG2a (µg/ml) | IgG3 (µg/ml) | IgM (µg/ml) |
|---|---|---|---|---|---|
| 10th week | |||||
| − | − | 3 | 557 ± 145 | 863 ± 298 | 368 ± 66 |
| − | + | 7 | 12 695 ± 1698 | 4966 ± 645 | 3244 ± 533 |
| + | − | 4 | 407 ± 209 | 413 ± 46 | 177 ± 19 |
| + | + | 6 | 2414 ± 252 | 1731 ± 355 | 1499 ± 290 |
| 13th week | |||||
| − | − | 3 | 491 ± 5 | 213 ± 48 | 364 ± 67 |
| − | + | 5 | 12 071 ± 1140 | 2986 ± 362 | 2412 ± 432 |
| + | − | 3 | 171 ± 76 | 233 ± 80 | 349 ± 31 |
| + | + | 5 | 2037 ± 633 | 790 ± 184 | 992 ± 148 |
Dosages were performed on sera from individual mice, according to the protocol described in the Materials and Methods. Values given in this table, expressed in µg/ml, represent the arithmetic means of individual values ± SEM
Phenotypic abnormalities
Phenotypic abnormalities associated with MAIDS include the expansion of large B cells expressing B220 at low density,6 and the expansion of the Thy‐1.2– CD4+ subset.23,24 At the 13th week, fluorescence‐activated cell sorter (FACS) analysis confirmed these phenotypic shifts in each LN suspension from non‐transgenic infected mice (not shown). By contrast, in LN suspensions of infected transgenics the blastic shift was inconstantly detected and, when present, involved only a small fraction of the total B population than in non‐transgenics, and there was no expansion of the Thy‐1.2– CD4+ subset (not shown).
Semi‐quantitative evaluation of cytokine gene expression
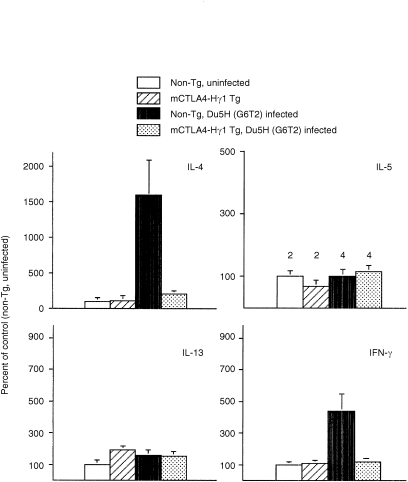
Among the various cytokines tested, only transcripts encoding sequences specific for IL‐4, – 5, – 13 and IFN‐γ were detected in both PBS‐and Du5H injected mice (Fig. 3). The levels of transcripts in uninfected transgenic and nontransgenic mice did not significantly differ. At the 13th week after viral inoculation, the main findings in non transgenic mice were a 15‐fold increase of IL‐4 transcripts and a 5‐fold increase of IFN‐γ transcripts whereas the levels of IL‐13 only slightly increased. By sharp contrast, in infected mCTLA4Hγ1 transgenic mice, no upregulation of IFN‐γ was detected and the level of IL‐4 mRNA was only twice of that in uninfected controls (Fig. 3).
Figure 3.
Measurements of cytokine mRNA levels with the RNAse protection assay. The assays were performed on RNA extracts from SP samples of individual experimental mCTLA4‐Hg1 tg and non‐tg mice at the 13th week after infection with Du5H (G6T2) or sham inoculation with PBS. The number of mice studied in each group is indicated over the top of the corresponding column. Selected film exposures yielding similar overall signal intensities between experiments were scanned with a densitometer (PhosphorImager) to obtain absorbance values for cytokines and L32 transcripts. Quantification of cytokine mRNA levels was done by normalization to those of the L32 housekeeping gene. The values for infected non‐tg, infected tg and uninfected tg mice were converted to percentage of values obtained in uninfected non‐tg mice. The mean indexes (± SEM) from all experiments are shown.
Defective viral gene expression
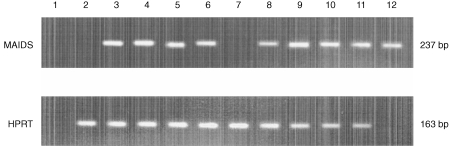
The mRNAs prepared from spleens removed at the 10th week were examined by RT‐PCR for expression of defective virus (Fig. 4). No transcripts were detected in any of the non‐infected mice. Du5H was demonstrated in every infected animal; when semi‐quantitatively compared to HPRT expression, no difference in the level of Du5H expression was found between mCTLA4‐Hγ1 transgenics and non‐transgenics (Fig. 4).
Figure 4.
Relative expression of gag mRNA is identical in infected mCTLA4‐Hg1 tg and non‐tg littermates. The cDNA obtained from reaction of spleen RNA samples of individual mice with RT were amplified by either defective gag‐ or HPRT‐specific primers for 30 cycles. The bands for the two products appeared on a 2% agarose gel at the expected migration points corresponding to 237 and 163 bp, respectively. Lanes 2 and 7 are, respectively, from uninfected non‐tg and tg mice. Lanes 3–6 are from infected non‐tg mice at either the 10th (lanes 3 and 4) or the 13th week (lanes 5 and 6) after viral inoculation. Lanes 8–11 are from infected tg mice at the 10th (lanes 8 and 9) or the 13th week (lanes 10 and 11) after viral inoculation. Lane 1 is a negative control for PCR (water). Lane 12 is a specificity control for PCR using J1 plasmid containing the sequence of Du5H. No amplification was obtained after PCR reaction of RNAs samples (not shown).
Discussion
Pathogenesis of MAIDS clearly implies interactions between B and CD4+ T cells.7 The present work addressed the question of a possible involvement of CTLA4‐CD28/B7 counter‐receptors interactions in MAIDS development. Our results clearly demonstrate that mCTLA4‐Hγ1 transgenic mice do not develop the cardinal features of MAIDS even several months after repeated inoculations of the MAIDS virus.
The mCTLA4‐Hγ1 transgenics were initially produced from (C57BL/6 × DBA/2)F1 backcrossed with C57BL/6 mice. Since DBA/2 mice are resistant to MAIDS,25 the hypothesis of a non‐permissive genetic background of the tg mice must be addressed. This hypothesis can be formally ruled out for the following reasons: first, in F1 crosses between resistant and sensitive strains, sensitivity to disease is dominant, rather than resistance;25 second, the infected transgenic mice display high expression of defective MAIDS virus, a finding which correlates with genetic susceptibility to MAIDS;25 third, the non‐transgenic controls have the same genetic background and are fully susceptible to the disease.
Expression of mCTLA4‐Hγ1 is clearly associated with a much more complete and sustained protection than that obtained by iterative i.p. injections of CTLA4Ig.14 This finding is likely due to a higher concentration of the fusion protein in tg mice. Despite the lack of pharmacodynamic data about the fate of injected mCTLA4Ig in mice, it can be reasonably inferred from studies on serum clearance of huCTLA4Ig injected intravenously (i.v.) into mice15 that the steady‐state concentration of the mCTLA4Ig obtained after repeated i.p. injections of 50 µg three times a week is probably close to 10 µg/ml. The serum levels of mCTLA4‐Hγ1 fusion protein achieved in tg mice are basically 10–30 µg/ml16 and can be assumed to be much higher in mice with MAIDS where the transgene was found to be expressed by a very large number of blastic plasmacytoid B cells in the spleen (not shown).
Alternatively, the phenotype of mCTLA4‐Hγ1 mice could also contribute to their resistance to the disease, since these mice are devoid of germinal centres.16 Interestingly, MAIDS is associated with the dramatic expansion of B and T cells with rare phenotypes normally restricted to germinal centres (B220dim B cells26 and T CD4+ Thy‐1– cells27). This suggests that the abnormal interactions between B cells and CD4+ T cells leading to MAIDS could preferentially take place in germinal centres or in a functionally similar microenvironment. Despite a high viral gene expression in the lymphoid organs of the infected mCTLA4‐Hγ1 mice, there is only a very limited expansion of B220dim B cells in these animals. This could indicate that the B‐cell subset which mediates the abnormal interactions with CD4+ T cells and induces their MAIDS‐associated functional defects is absent or reduced in mCTLA4‐Hγ1 mice. A much lower frequency of ‘MAIDS‐inducing’ B cells (due to absence of GC) could therefore permit a higher protective effect of a given concentration of CTLA4Ig. Such a mechanism could also contribute to the resistance to MAIDS of other strains of mice deficient in MHC class II8 or CD4028 ‐deficient mice to MAIDS since these animals are also devoid of GC.29,30
Despite their resistance to MAIDS, infected mCTLA4‐Hγ1 mice display similar Du5H viral gene expression to infected non‐transgenic mice. Similar findings have recently been reported in CD40‐deficient mice infected with LP‐BM5def: the absence of CD40 does not change the expression level of the defective virus.28 These obervations demonstrate that the infection of target cells by the defective virus and its persistence in this population is not strictly dependent on cognate T‐cell help; furthermore they suggest that a large fraction of non‐infected cells must participate in the lymphoproliferative process which develops in the infected non‐transgenic mice. Despite the fact that Du5H viral gene expression analysis is relative and not absolute (i.e. mRNA is prepared from the same number of lymphoid cells in tg and non‐tg animals), a strong proliferative advantage of infected cells over non‐infected cells in the non‐transgenic mice would have led to a higher viral gene expression in the latter animals.
Another important issue, which was not addressed here, is the expression of the Pr60gag protein in the infected mCTLA4‐Hγ1 tg mice. Type 1‐like cytokines have been involved in human immunodeficiency virus type 1 (HIV‐1) post‐transcriptional activation,31 it is therefore conceivable that mCTLA4‐Hγ1 tg mice which secrete less IFN‐γ than their non‐transgenic counterparts in response to retroviral infection are characterized by much lower Pr60gag protein expression despite a similar relative viral gene expression.
The mCTLA4‐Hγ1 tg mice have poor antibody class switching in response to T‐dependent antigens, in correlation with the absence of GC development.16 In our model, mCTLA4‐Hγ1 did not prevent infected mice from developing slight hypergammaglobulinaemia, involving both the IgM and IgG isotypes. Whereas CD28 is required for germinal centre reactions, the classical site for T‐dependent antibody responses, MAIDS‐associated hypergammaglobulinaemia may partly depend on other extrafollicular B‐cell differentiation pathways, possibly independent from CD28/B7‐mediated signals. A previous report on antiviral immune responses in mCTLA4‐Hγ1 transgenic mice suggested that highly replicating viruses, such as lymphocytic choriomeningitis virus (LCMV), might compensate for the decrease in T help caused by the blocking effects of soluble CTLA4.32 Recently, the heat‐stable antigen (HSA) was shown to be involved in the antigen‐induced immunoglobulin class switch occurring in mice deficient in CD28.33
The efficacy of CTLA4Ig fusion protein for the inhibition of certain auto‐immune diseases for the prevention of graft rejection has been largely ascribed to a modulation of cytokine secretion, most often a reversal from a T helper type 1 (Th1) to a Th2 pattern.34,34 Interestingly, in the case of MAIDS infection, the resistance of the mCTLA4‐Hg1 tg mice is associated with an almost complete abrogation of the up‐regulation of both IFN‐γ (a Th1 cytokine) and IL‐4 (a Th2 cytokine) normally found in susceptible nontg mice. Whereas it was claimed several years ago that IL‐4 plays a major role in MAIDS pathogenesis,35 subsequent studies favoured the hypothesis that high‐level expression of this cytokine was an epiphenomenon of disease.36 If this holds true, a low level of expression of IL‐4 in infected tg mice was rather expected. The role played by IFN‐γ in MAIDS is complex: several studies have shown that constitutive expression of this cytokine contributes to the progression of the syndrome. Accordingly, mice deficient in IFN‐γ or treated with a neutralizing antibody to IFN‐γ develop disease with a delayed time–course.37 Surprisingly, overexpression of this cytokine by treatment with high‐dose IL‐12 renders mice resistant to disease.38 It was therefore proposed that at high concentrations, IFN‐γ can directly inhibit B‐cell proliferation and limit the development of MAIDS.37 Since treatment with CTLA4Ig has been shown to increase IFN‐γ secretion in several models characterized by an in vivo Th2 switch,39,40 it was interesting to assess the secretion of this cytokine in the infected transgenic animals.
Our results clearly show that the disease resistance conferred by mCTLA4‐Hγ1 tg expression is certainly not linked to a protective effect of IFN‐γ overexpression. Further experiments consisting of administration of rIFN‐γ to mCTLA4‐hγ1 tg mice may possibly contribute to a better understanding of the role of this cytokine in MAIDS.
Several models of T‐cell anergy are characterized by incomplete signalling (i.e. TCR occupancy without appropriate costimulatory signals). Although previous reports have suggested that MAIDS could be due to similar mechanisms, Andrews et al. recently demonstrated that anergy induced by LP‐BM5 infection cannot be reversed by providing costimulation via CD28 and IL‐12.41 Our results are quite compatible with this finding and suggest that at some point a complete activation of T cells must occur. T‐cell refractoriness in vitro could therefore reflect chronic overactivation rather than an incomplete, or intrinsically abnormal activation process.
Acknowledgments
We thank Ms M.C. Petit, Mrs Delory and Ms F. Nizol (Experimental Immunology Unit, University of Louvain, Belgium) for their excellent technical assistance. We wish to acknowledge Professor P. Jolicoeur for providing the virus and Professor H. Bazin for providing murine isotype‐specific antibodies for ELISA assays. This work was supported in part by the Fund for Scientific Medical Research (FRSM) and the Centre Anticancéreux près l'Université de Liège. L.dL, S.D. and M.M. are, respectively, research assistant, senior research assistant and research associate of the Fonds National de la Recherche Scientifique (FNRS).
References
- 1.Duplan JF, Monnot P, Mistry PB. [Leukemogenic activity of the mouse radioleukosis virus] Bull Cancer. 1970;57:23. [PubMed] [Google Scholar]
- 2.Legrand E, Daculsi R, Duplan JF. Characteristics of the cell populations involved in extra‐thymic lymphosarcoma induced in C57BL/6 mice by RadLV‐Rs. Leuk Res. 1981;5:223. doi: 10.1016/0145-2126(81)90107-7. [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay SK, Morse HC, Makino M, Ruscetti SK, Hartley JW. Defective virus is associated with induction of murine retrovirus‐induced immunodeficiency syndrome. Proc Natl Acad Sci USA. 1989;86:3862. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz DC, Hanna Z, Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989;338:505. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- 5.Mosier DE, Yetter RA, Morse HC. Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985;161:766. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang M, Simard C, Kay DG, Jolicoeur P. The majority of cells infected with the defective murine AIDS virus belong to the B‐cell lineage. J Virol. 1991;65:6562. doi: 10.1128/jvi.65.12.6562-6571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yetter RA, Buller RM, Lee JS, et al. CD4+ T cells are required for development of a murine retrovirus‐induced immunodeficiency syndrome (MAIDS) J Exp Med. 1988;168:623. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giese NA, Giese T, Morse HC. Murine AIDS is an antigen‐driven disease: requirements for major histocompatibility complex class II expression and CD4+ T cells. J Virol. 1994;68:5819. doi: 10.1128/jvi.68.9.5819-5824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makino M, Yoshimatsu K, Azuma M, et al. Rapid development of murine AIDS is dependent of signals provided by CD54 and CD11a. J Immunol. 1995;155:974. [PubMed] [Google Scholar]
- 10.Green KA, Crassi KM, Laman JD, et al. Antibody to the ligand for CD40 (gp39) inhibits murine AIDS‐associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease‐susceptible C57BL/6 mice. J Virol. 1996;70:2569. doi: 10.1128/jvi.70.4.2569-2575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green KA, Noelle RJ, Green WR. Evidence for a continued requirement for CD40/CD40 ligand (CD154) interactions in the progression of LP‐BM5 retrovirus‐induced murine AIDS. Virology. 1998;241:260. doi: 10.1006/viro.1997.8970. [DOI] [PubMed] [Google Scholar]
- 12.Boussiotis VA, Freeman GJ, Gribben JG, Nadler LM. The role of B7/B7: CD28/CLTA‐4 pathways in the prevention of anergy, induction of productive immunity and down‐regulation of the immune response. Immunol Rev. 1996;153:5. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 13.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 14.De Leval L, Colombi S, Debrus S, et al. CD28–B7 costimulatory blockade by CTLA4Ig delays the development of retrovirus‐induced murine AIDS. J Virol. 1998;72:5285. doi: 10.1128/jvi.72.6.5285-5290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA‐4 T cell activation molecule. Science. 1992;257:792. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 16.Lane P, Burdet C, Hubele S, et al. B cell function in mice transgenic for mCTLA4‐H gamma 1: lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J Exp Med. 1994;179:819. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Simard C, Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper‐free defective retrovirus. Science. 1989;246:1614. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- 18.Rowe WP, Pugh WE, Hartley JW. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Fujiwara M. [Exocrinopathy resembling Sjogren's syndrome induced by a murine retrovirus] Nippon Rinsho. 1995;53:2461. [PubMed] [Google Scholar]
- 20.Svetic A, Finkelman FD, Jian YC, et al. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391. [PubMed] [Google Scholar]
- 21.Sassen A, Vander PF, Janowski M, Maisin JR. Immunoglobulin analysis of C57BL mice infected with a radiation‐induced leukemia virus. J Natl Cancer Inst. 1974;52:539. doi: 10.1093/jnci/52.2.539. [DOI] [PubMed] [Google Scholar]
- 22.Pattengale PK, Taylor CR, Twomey P, et al. Immunopathology of B‐cell lymphomas induced in C57BL/6 mice by dualtropic murine leukemia virus (MuLV) Am J Pathol. 1982;107:362. [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes KL, Morse HC, Makino M, Hardy RR, Hayakawa K. A unique subset of normal murine CD4+ T cells lacking Thy‐1 is expanded in a murine retrovirus‐induced immunodeficiency syndrome, MAIDS. Eur J Immunol. 1990;20:2783. doi: 10.1002/eji.1830201237. [DOI] [PubMed] [Google Scholar]
- 24.Moutschen MP, Colombi S, Deprez M, et al. Population dynamics of CD4+ T cells lacking Thy‐1 in murine retrovirus‐induced immunodeficiency syndrome (MAIDS) Scand J Immunol. 1994;39:216. doi: 10.1111/j.1365-3083.1994.tb03363.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang M, Simard C, Jolicoeur P. Susceptibility of inbred strains of mice to murine AIDS (MAIDS) correlates with target cell expansion and high expression of defective MAIDS virus. J Virol. 1992;66:2398. doi: 10.1128/jvi.66.4.2398-2406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell‐dependent and T cell‐independent antigens [published erratum appears in Eur J Immunol 1992 February; 22 (2): 615] Eur J Immunol. 1991;21:2951. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 27.Zheng B, Han S, Kelsoe G. T helper cells in murine germinal centers are antigen‐specific emigrants that downregulate Thy‐1. J Exp Med. 1996;184:1083. doi: 10.1084/jem.184.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu P, Morawetz RA, Chattopadhyay S, Makino M, Kishimoto T, Kikutani H. CD40‐deficient mice infected with the defective murine leukemia virus LP‐BM5def do not develop murine AIDS but produce IgE and IgG1 in vivo. Eur J Immunol. 1999;29:615. doi: 10.1002/(SICI)1521-4141(199902)29:02<615::AID-IMMU615>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Cardell S, Merkenschlager M, Bodmer H, et al. The immune system of mice lacking conventional MHC class II molecules. Adv Immunol. 1994;55:423. doi: 10.1016/s0065-2776(08)60515-5. [DOI] [PubMed] [Google Scholar]
- 30.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40‐deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 31.Al Harthi L, Roebuck KA, Landay A. Induction of HIV‐1 replication by type 1‐like cytokines, interleukin (IL) ‐12 and IL‐15: effect on viral transcriptional activation, cellular proliferation, and endogenous cytokine production. J Clin Immunol. 1998;18:124. doi: 10.1023/a:1023246800353. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann C, Seiler P, Lane P, Zinkernagel RM. Antiviral immune responses in CTLA4 transgenic mice. J Virol. 1997;71:1802. doi: 10.1128/jvi.71.3.1802-1807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Zhou Q, Zheng P, Liu Y. CD28‐independent induction of T helper cells and immunoglobulin class switches requires costimulation by the heat‐stable antigen. J Exp Med. 1998;187:1151. doi: 10.1084/jem.187.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury SJ, Akalin E, Chandraker A, et al. CD28–B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521. [PubMed] [Google Scholar]
- 35.Kanagawa O, Vaupel BA, Gayama S, Koehler G, Kopf M. Resistance of mice deficient in IL‐4 to retrovirus‐induced immunodeficiency syndrome (MAIDS) [see comments] Science. 1993;262:240. doi: 10.1126/science.8211142. [DOI] [PubMed] [Google Scholar]
- 36.Morawetz RA, Doherty TM, Giese NA, et al. Resistance to murine acquired immunodeficiency syndrome (MAIDS) [letter; comment] Science. 1994;265:264. doi: 10.1126/science.8023146. [DOI] [PubMed] [Google Scholar]
- 37.Morse HC, Giese N, Morawetz R, et al. Cells and cytokines in the pathogenesis of MAIDS, a retrovirus‐induced immunodeficiency syndrome of mice. Springer Semin Immunopathol. 1995;17:231. doi: 10.1007/BF00196167. [DOI] [PubMed] [Google Scholar]
- 38.Gazzinelli RT, Giese NA, Morse HC. In vivo treatment with interleukin 12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS) J Exp Med. 1994;180:2199. doi: 10.1084/jem.180.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padrid PA, Mathur M, Li X, et al. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol. 1998;18:453. doi: 10.1165/ajrcmb.18.4.3055. [DOI] [PubMed] [Google Scholar]
- 40.Shanafelt MC, Kang I, Barthold SW, Bockenstedt LK. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T‐ cell costimulatory pathway. Infect Immun. 1998;66:266. doi: 10.1128/iai.66.1.266-271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrews C, Swain SL, Muralidhar G. CD4 T cell anergy in murine AIDS: costimulation via CD28 and the addition of IL‐12 are not sufficient to rescue anergic CD4 T cells. J Immunol. 1997;159:2132. [PubMed] [Google Scholar]