Abstract
Conventional vaccination with the cocaine molecule conjugated to a protein carrier is a new approach in the treatment of addiction. Experimentally, this strategy has been shown to alter the pharmacokinetics as well as the psychostimulant effect of a cocaine challenge. The purpose of this study was to investigate whether a more stable and less controversial molecule, an anti-idiotypic antibody, which mimics the configuration of the cocaine molecule (Ab2β), could be successfully used instead of cocaine. Two cocaine conjugates that presented different areas of the cocaine molecule to the immune system were used to produce monoclonal antibodies specific for cocaine (Ab1). Several anti-idiotypic antibodies were then produced. Four were identified as Ab2β, or internal images of the antigen; when injected into BALB/c mice, they elicited an anticocaine response. The anticocaine response elicited by one of the four Ab2β (K1–4c) was sufficient to significantly reduce the level of cocaine that targeted the brain following cocaine challenge, compared with the level of cocaine found in the brain of control animals immunized with irrelevant antibody. In conclusion, the possibility of an anti-idiotypic vaccine seems to be worth pursuing.
Introduction
Cocaine is currently one of the most widespread illicit drugs in the United States and a major public health problem in industrialized countries. Current pharmacological and psychological therapies for the treatment of cocaine addiction have met with little success. Thus, new therapies should be investigated.
The synthesis and release of catecholamines, such as dopamine, in the synaptic cleft of the mesolimbic region of the brain is responsible for neurotransmission. One of the primary mechanisms of clearing catecholamines from the synaptic cleft and of regulating their action is through reuptake of the neurotransmitters. In the brain, the cocaine molecule binds the catecholamine reuptake transporters, thus blocking catecholamine reuptake. This results in an increase in the level of catecholamines in the synaptic cleft, enhancing neurotransmission and triggering the psychoactive effects of cocaine. The extremely rapid rise of dopamine levels in the brain resulting from cocaine's targeting of that organ causes the very intense psychoactive effects and is thought to be the reason for the strongly addictive nature of cocaine.1 As effective pharmacotherapeutic agents suitable for counteracting cocaine craving and consequent relapse are not available, other strategies must be sought.
A novel approach to the treatment of cocaine addiction involves active immunization of patients. In fact, cocaine-specific antibodies present in the circulation have been found to bind cocaine, preventing it from entering the central nervous system (CNS) through the blood–brain barrier.2–4 This approach has numerous advantages over conventional therapies. Active immunization against cocaine will have fewer side-effects than pharmacotherapies, which alter brain chemistry. Furthermore, this treatment as part of a rehabilitation programme would not interfere with alternative peripheral cocaine-blocking agents or pharmacotherapies, which could be administered concurrently.
Research on the use of antibodies to block the effects of drugs dates back to 1974, when Bonese et al. successfully vaccinated a rhesus monkey against opiate self-administration.5
Recently, Carrera et al.2 found that immunization of male Wistar rats with a cocaine–keyhole limpet haemocyanin (KLH) conjugate ‘suppressed locomotor activity and stereotyped behaviour induced by cocaine, but not by amphetamine.’ The immunized animals exhibited a 42% decrease in the psychostimulant effect of cocaine as compared to control animals and displayed lower levels of cocaine in the striatum (52%) and cerebellum (77%) than did the control animals.
In their studies, Fox et al.3 trained BALB/c mice to self-administer 1 mg/kg of cocaine per intravenous (i.v.) infusion. After vaccination with a cocaine–bovine serum albumin (BSA) conjugate, the mice decreased self-administration of cocaine to background levels. Immunized mice also displayed a significant change in cocaine pharmacokinetics with decreased levels of cocaine measured in the brain. The same researchers also determined that the metabolism of cocaine in vivo was not altered by the antibody binding. The ratio of cocaine to its metabolites in the plasma of cocaine-immunized mice was comparable with that of the control mice. Chronic administration of cocaine did not appear to affect the ability of the vaccine to induce cocaine-specific antibodies. Such immunization also seemed to reduce the psychoactive effects of cocaine, even when cocaine was administered in large doses.3,6
Ettinger et al. produced a cocaine–KLH conjugate for immunization of female Long-Evans rats.4 They found that the cocaine–KLH conjugate elicited antibodies specific for cocaine and that this antibody response was sufficient to cause a change in the behaviour of cocaine-challenged animals.
Another entirely different approach to the immunological control of cocaine use involved catalytic antibodies capable of causing cocaine to be degraded. Catalytic antibodies, by binding a transition state of a chemical reaction, catalyze that reaction in the same manner as enzymes.7 Two groups have reported that a stable analogue of the unstable transition state of hydrolysis of the benzoylester side group of cocaine can serve as a hapten for the production of catalytic antibodies.8,9 The antibodies catalyze cleavage of the benzoylester, yielding the inactive metabolites ecgonine methylester and benzoic acid.9 Passive immunization with such a catalytic antibody could provide a treatment for dependence by blunting reinforcement; however, the catalytic activity of the antibodies produced thus far is not sufficient to produce clinical changes.
To date, all experimental cocaine vaccines utilize, as immunogens, cocaine analogues linked to a carrier protein. This is the most straightforward approach; however, a seldom discussed but acknowledged caveat to these vaccines is the instability of the cocaine analogues linked to the carrier protein.2 To circumvent the problems associated with this instability, we propose the use of an anti-idiotypic vaccine, which uses as immunogen an antibody molecule, the configuration of which mimics the configuration of the cocaine molecule. According to Jerne's network theory10 an antibody specific for an antigen (idiotypic antibody or Ab1) elicits different sets of anti-idiotypic antibodies (Ab2), which can bind specifically to the various idiotopes on the original Ab1 molecule. One of these sets, the Ab2β, binds idiotopes within the antigen-combining site of the Ab1, mimicking the configuration of the antigen, and is referred to as ‘the internal image of the antigen.’ Internal image-bearing Ab2βs have been demonstrated in several antigenic systems and can elicit specific antibody responses (Ab3) similar to the Ab1 antibodies induced by the antigen. Anti-idiotypic vaccines use Ab2β as immunogen rather than the original antigen. Although the development of anti-idiotypic vaccines has not been as successful as anticipated at the time of their conceptual formulation, numerous investigations of the use of Ab2βs for vaccination against infectious agents, such as human hepatitis virus,11 human immunodeficiency virus (HIV),12Neisseria meningitidis,13Chlamydia trachomatis,14 as well as tumour-associated antigens15 and lipopolysaccharide (LPS) inner-core determinants,16 are presently ongoing, and several are undergoing clinical trials.17,18
In the present study, we used a number of monoclonal antibodies (mAbs) induced with two different cocaine–KLH conjugates and produced a panel of Ab2 mAbs that showed the distinct characteristics of Ab2β. In fact, one, when used to vaccinate mice, induced an anticocaine response sufficient to reduce the level of cocaine targeting the brain following cocaine challenge.
Materials and methods
Animals
BALB/c mice were used throughout this work. They were purchased from Harlan-Sprague Dawley (Indianapolis, IN) and housed in the animal facilities at the College of Veterinary Medicine. To elicit Ab1 or Ab2 Abs, mice were injected intraperitoneally (i.p.) with 100 μg of the desired cocaine–KLH or Ab1–KLH conjugate emulsified in Freund's complete adjuvant (FCA), and at 4-week intervals thereafter with 100 μg of the antigen emulsified in Freund's incomplete adjuvant (FIA), until a significant serum titre was obtained by enzyme-linked immunosorbent assay (ELISA). In the final experiments, mice were immunized i.p. with Ab2β–KLH in alum.
Synthesis of cocaine hapten precursor
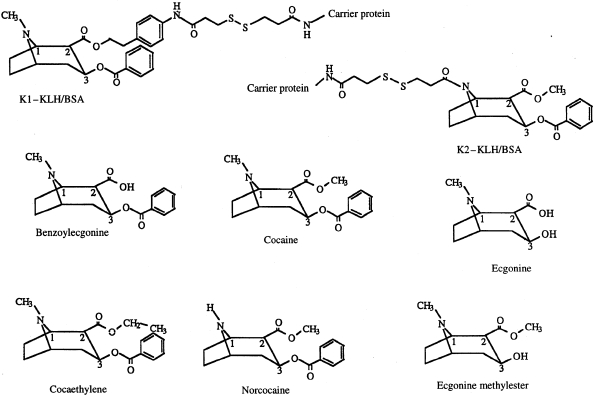
Two cocaine hapten precursors, K1 and K2 (Fig. 1), were prepared from the pharmacologically active cocaine derivative 3β-(benzoyloxy)-8-azabicyclo[3.2.1]octaine-2β carboxylic acid (p-aminophenyl) ethyl ester,19 that contains an aromatic amino group and from norcocaine,20 which contains a secondary amine. A mixture of the appropriate cocaine derivative (0·02 mmol) and SPDP (N-succinimidyl-3-[2-pyridyldithio] propionate; Pharmacia, Piscataway, NJ) (0·20 mmol) was dissolved in ethanol (1 ml), and phosphate buffer (pH 7·4, 1 ml) was then added. The reaction was stirred at room temperature for 1 hr. Ethanol was removed under reduced pressure and the product was extracted in CH2C12. Volatiles were evaporated to afford an oily residue, which was purified by flash chromatography (hexanes: acetone) resulting in a yield of ≈ 60–70%. These precursors, which still contain a reactive functional group, were used for conjugation to the protein carrier.
Figure 1.
Chemical structures (formulas) of the cocaine conjugates (K1-protein and K2-protein) together with those of cocaine and its principal metabolites.
Antigens (cocaine conjugates and antibody conjugates)
Both cocaine hapten precursors K1 and K2 were linked to KLH and BSA, again using the heterobifunctional linker SPDP, following the manufacturer's specifications. Glutaraldehyde was used for the conjugation of Ab1 to KLH.21
Production of hybrids
Fusion procedures followed the technique of Oi & Herzenberg,21 as routinely performed in our laboratory.22 Six fusions were performed with K1–KLH-immunized mice; five fusions were performed with K2–KLH-immunized mice. The selected positive wells were cloned twice by limiting dilution. mAb production and purification followed the techniques described by Mueller et al.23 and Reik et al.24 The purity of mAb was assessed by gel electrophoresis.
Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay (ELISA) basic technique followed the description of Muhumuza et al.22 Sera and hybrid supernatants were screened for Ab1 on plates coated with the immunizing cocaine hapten precursor linked to BSA, and developed with goat anti-mouse immunoglobulin (H & L) conjugated to horseradish peroxidase (HRP) (Southern Biotechnology Associates, Inc., Birmingham, AL). For initial screening of the specificity of binding, mAb1 were incubated for 90 min with soluble antigen (cocaine-HCl) before reaction with the coated plate. Screening for Ab2 was performed using two methods: sera and hybrid supernatants were reacted on plates coated with the immunizing mAb1; the same immunizing mAb1 in biotinylated form was then used as a developing reagent followed by ExtrAvidin peroxidase conjugate (Sigma, St. Louis, MO) and substrate. As the coating and the developing antibody are the same, and they both bind the same idiotope on the mAb2 molecule, the ratio between the coating mAb1 and the mAb2 in the supernatants is critical. After reacting with the mAb1 coating the plate, sufficient sites of the mAb2 molecules need to be available for the developing biotinylated mAb1 to bind. In the alternative detection method, sera and supernatants were reacted on plates coated with F(ab′)2 fragments of the immunizing mAb1; the developing system consisted of HRP-labelled goat anti-mouse immunoglobulin G (IgG) Fc fraction (Accurate Chemical, Westbury, NY) or HRP-labelled protein G (Sigma-Aldrich, St. Louis, MO) and 2.2′-azino-di[3-ethyl-benzthiazoline sulfon-ate (6)] (ABTS) substrate. This technique proved to be both quicker and more sensitive than the first method and thus was used in all subsequent screenings. The similarity of mAb2 to the cocaine molecule was tested by an inhibition assay. Fifty microlitres/well of soluble antigen (cocaine-HCl) was incubated on the plate coated with the immunizing mAb1. After washing the plate, Ab2β-containing serum dilutions or supernatants were added and the assay was completed as described above. Sera were screened for Ab1-like Ab3 responses using the method previously described for Ab1-containing sera and supernatants. The determination of the affinity constant (Ka) was as described by Friguet et al.25 and as previously performed in our laboratory22 using a range of cocaine-HCl concentrations between 500 μg/ml and 1 ng/ml.
Production of F(ab′)2 fragments
Pepsin fragmentation of the various mAbs was carried out according to the technique described in reference 26.
Extraction and evaluation of cocaine from brain tissue
Mice were fasted for 15 hr prior to challenge with cocaine and brought to the laboratory in their original cage. Five mg/kg of cocaine-HCl was injected i.p. in 200 μl of saline. After an interval of 10 min (previously determined to allow the maximum concentration of cocaine to reach the brain), the animal was killed by cervical dislocation, and the brain was collected, weighed and immediately frozen in a 1·5-ml polypropylene conical tube in an ethanol/dry ice bath. All samples were stored at − 70° until the day of extraction. Thawed brains were homogenized on ice in 5 ml of 0·1 m phosphate buffer, pH 6·0, using a Dounce homogenizer. Sixty microlitres of the HPLC internal standard bupivacaine (0·1 mg/ml) was added and the sample incubated on ice for 8 min. The homogenates were centrifuged at 7000 g for 10 min at 5°. The supernatant was removed and 2 ml of phosphate buffer was added to the pellet, which was vortexed and recentrifuged. The supernatants were again removed, pooled and added to a conditioned solid-phase extraction (SPE) column. Bond Elut Certify (Varian, Harbor City, CA) mixed-phase extraction columns (200 mg sorbent bed 10 ml) were used for extraction of cocaine, according to the manufacturer's specifications.
HPLC methods
Samples from SPE were reconstituted with 300 μl of the mobile phase (82% 0·1 m potassium phosphate buffer, pH 2·7, and 18% acetonitrile containing 0·5% triethylamine). One hundred microlitres was injected twice using a gas-tight Hamilton syringe. The system was run isocraticly at 2 ml/min with an Adsorbosphere HS C18 (250 mm × 4·7 mm) column from Alltech (Chicago, IL). System Gold V810 software was used to control two 114M Beckman solvent delivery modules through a 406 analogue interface. A Spectroflow 783 programmable absorbance detector (Kratos Analytical Instruments) was used at 235 nm for the detection of cocaine and its metabolites. The retention times for benzoylecgonine (BE), cocaine, norcocaine and bupivacaine were ≈ 6·5, 12, 14 and 20 min, respectively. During every HPLC run the internal standard bupivacaine and cocaine-HCl (Sigma-Aldrich, St. Louis, MO) were analysed on the HPLC to prevent error caused by stock solution variation.
Calculations and statistical analysis
A standard curve for cocaine concentration was produced with the cocaine standard, and the concentration of cocaine in unknown samples was determined from the curve. Concentrations of all samples were normalized using the data from the internal standard (bupivacaine). Averages with the standard error (SE) were calculated using 512+ StatView™ (BrainPower, Inc., Calabas, CA). Statistical analysis was also performed with StatView™ using a one-tailed unpaired t-test.
Results
Description of the cocaine conjugates used to elicit anticocaine antibody (Ab1)
Two immunogenic conjugates of cocaine, K1–KLH and K2–KLH, were used (see the Materials and methods). Both conjugates contain a spacer (SPDP) between the cocaine moiety and the protein carrier molecule (Fig. 1). K1–KLH presents to the immune system the N-methyl and benzoyl groups, enhancing the likelihood of their recognition by the antibody, while K2–KLH presents a different side of the molecule, which allows for recognition of the methylester in the 2β-position and the benzoyl group in the 3β-position (Fig. 1). The stereochemical integrity at the 2β and 3β positions was verified during the synthesis of the two derivatives, as well as after preparation of the cocaine hapten precursor molecules, using proton nuclear magnetic resonance (NMR). This ensured an accurate representation of the native molecule's absolute configuration to the immune system.
The conjugates were also examined to determine the level of haptenation. This was performed using mass spectral analysis and the fluorescamine substitution assay. The fluorescamine assay indicated that the BSA conjugates contained a range of 10–29 hapten molecules/BSA while the KLH conjugates were found to contain from eight to 19 molecules of hapten/100 000 MW of KLH. Calculations based on mass spectral analysis indicated a substitution ranging from 12 to 14 K1 molecules per BSA and three to 11 K2 molecules per BSA. Owing to the high heterogeneity of the molecular weight of KLH, mass spectrometry was not possible on KLH conju-gates. Positive ELISA reactivity of these conjugates with commercially available anticocaine sera confirmed that cocaine was present on the carrier protein.
Induction and characterization of mAbs specific for cocaine (mAb1)
Three anti-K1–KLH and five anti-K2–KLH monoclonal Ab1 whose binding to their respective conjugates was inhibited by cocaine-HCl were selected, cloned and isotyped (Table 1). These antibodies were further characterized in order to ensure that the antigen-combining site of the Ab1, which would be used as antigen to elicit Ab2β, presented an accurate complementarity to the cocaine molecule. They were allowed to react with both the homologous and heterologous cocaine conjugates (Table 1). Their affinities versus cocaine, as well as its major metabolites, were measured on plates coated with the homologous cocaine conjugates (Table 2).
Table 1.
Characteristics of anticocaine monoclonal antibodies (mAb1) obtained from the spleen of BALB/c mice immunized with cocaine conjugates K1–KLH or K2–KLH
| K1–BSA | K2–BSA | ||||||
|---|---|---|---|---|---|---|---|
| Immunogen | Ab1* clones | Isotype | Affinity (Ka)§ | Reactivity† | Inhibitor‡ | Reactivity† | Inhibitor‡ |
| K1–KLH | Kl–1 | κγ1 | 5·9 × 106 | + | + | – | n/a |
| K1–2 | κγ1 | 5·9 × 104 | + | + | + | + | |
| K1–4 | κγ1 | 3·8 × 106 | + | + | – | n/a | |
| K2–KLH | K2–1 | λγ1 | 1·0 × 106 | + | + | + | + |
| K2–2 | κγ1 | 4·0 × 107 | – | n/a | + | + | |
| K2–3 | κγ1 | 2·0 × 108 | – | n/a | + | + | |
| K2–4 | κγ1 | 6·7 × 105 | + | + | + | + | |
| K2–5 | λγ2a | 2·0 × 106 | + | + | + | + | |
mAb1 are identified with the designation of the cocaine hapten precursor that elicited them (K1 or K2) followed by a number (K1–1, K1–2, etc., K2–1, K2–2, etc.).
Reactivity was determined by enzyme-linked immunosorbent assay (ELISA) on plates coated with K1–bovine serum albumin (BSA) or K2–BSA.
The inhibitor was soluble cocaine-HCl (1 mg/ml).
Affinity determination was performed with ELISA on plates coated with the homologous conjugate using, as inhibitor, a range of concentrations of cocaine-HCl between 500 μg/ml and 1 ng/ml.
Table 2.
Epitope specificity: reactivity and affinity of anticocaine monoclonal antibodies (mAbs), for the major cocaine metabolites
| Inhibitors* (Ka) | ||||||
|---|---|---|---|---|---|---|
| mAb1 | Ecgonine | Ecgonine methylester | Benzoyl ecgonine | Cocaethylene | Norcocaine | Cocaine |
| K1–1 | – | – | – | + (4·5 × 103)† | + (1·6 × 104) | + (5·9 × 106) |
| K1–2 | – | – | – | – | – | + (5·9 × 104) |
| K1–4 | – | – | – | + (1·2 × 104) | + (2·2 × 104) | + (3·8 × 106) |
| K2–1 | ND‡ | – | + (1·0 × 104) | + (3·0 × 104) | + (2·0 × 105) | + (1·0 × 106) |
| K2–2 | – | – | + (1·9 × 104) | + (1·1 × 106) | + (1·5 × 107) | + (4·0 × 107) |
| K2–3 | – | – | + (3·3 × 105) | + (2·0 × 107) | + (1·8 × 108) | + (2·0 × 108) |
| K2–4 | – | – | + (2·8 × 103) | + (1·4 × 105) | + (1·0 × 106) | + (6·7 × 105) |
| K2–5 | ND‡ | – | + (8·6 × 103) | + (2·4 × 104) | + (3·6 × 104) | + (2·0 × 106) |
The concentration range of each inhibitor was between 500 μg/ml and 1 ng/ml; the mAb1 concentrations were 100 ng/ml.
Affinity constant (in parenthesis) determination was performed on plates coated with the homologous conjugate.
As seen in Table 1, of the K1-specific antibodies, only K1–2 reacted with the heterologous K2–BSA conjugate and was inhibited with soluble cocaine. This indicates that the N-methyl group, which is not present on the K2–BSA conjugate, is important in the specificity of K1–1 and K1–4 antibodies. Indeed their affinity for norcocaine, which lacks the N-methyl group, was very low (Table 2). The K1–2 antibody had a very low affinity for its homologous conjugate. This ‘loose’ binding allows the cross-reactivity seen with the K2–BSA. Both the 2β methylester and the 3β benzoylester were found to be essential for binding of K1–1 and K1–4 antibodies because they did not bind BE or ecgonine methylester. This is somewhat contradictory in that the K1 conjugates are linked through the 2β methylester; however, binding still occurred with cocaethylene, which contains a transesterified 2β methyl group. This shows that as long as a molecule presents a methyl (methylene) group at the 2β position, these antibodies can bind it; in fact, this was the case with the K1–BSA conjugate. Thus, both K1–1 and K1–4 recognize the entire cocaine molecule. This is not surprising because the molecular weight of cocaine (303 Da) is close to the molecular weight of the space-filling fluorescein molecule (332 Da), which is known to fit completely into an antibody-combining site.27
Antibodies K2–1, K2–4 and K2–5, all with low affinities for the homologous conjugate K2–BSA, reacted with the K1–BSA conjugate. On the other hand, K2–2 and K2–3 antibodies, with high affinities for the homologous conjugate, displayed no reactivity with K1–BSA. This suggests that the 3β benzoylester group found on both K1 and K2 conjugates is important and sufficient for the binding of K2–1, K2–4 and K2–5 antibodies. The specificity of the K2–2 and K2–3 antibodies must also include the free methylester group, not present on the K1 conjugate, which may explain the lack of reactivity observed with K1–BSA. Whilst examining the reactivities of these antibodies with the cocaine metabolites, it was indeed found that the 3β benzoylester group was essential for the binding of all the K2-specific antibodies. This was concluded because all of the K2 specific antibodies displayed reactivity with cocaine, cocaethylene, norcocaine and BE, whilst not reacting with ecgonine methylester or ecgonine.
Examination of the affinities of all the K2-specific antibodies for the cocaine metabolites revealed that the 2β methylester appears to be involved, to a certain extent, in their binding. This was most evident in the case of K2–2 and K2–3 antibodies, whose affinities for BE (which lacks the 2β methylester group) were 1000-fold lower than for cocaine, and were 100-fold lower than for cocaethylene owing to the presence of a methyl group. Thus, antibodies K2–2 and K2–3, having high affinities for the homologous conjugate K2–BSA, bound very tightly to the cocaine molecule in the 2β methylester and 3β benzoylester region. These high-affinity antibodies did not bind K1–BSA owing to the steric hindrance of the linking region and possibly of the protein itself. This was not the case with K2–1, K2–4 and K2–5 antibodies, which were not only able to bind K1–BSA but also displayed similar reactivities with cocaethylene and BE, suggesting that the 2β-methylester is minimally involved in binding. As expected, the N-methyl group was not involved in the binding of the K2-specific antibodies as the K2 conjugates are linked through this group. This was concluded because their affinity for norcocaine (lacking the N-methyl group) is very similar to their affinity for cocaine.
Induction and production of Ab2β anti-idiotypic mAbs
Six mAb1 were purified, linked to KLH and used for the production of Ab2-secreting hybrids. Female BALB/c mice received injections of mAb1 antibodies K1–1, K1–2, K1–4, K2–1, K2–2, or K2–3. The presence of Ab2 antibodies in the sera of hyperimmunized mice, as well as in the supernatants of hybridomas after fusion, were verified by ELISA on plates coated with Ab1 F(ab′)2. For our purposes it was essential to distinguish between the different sets of Ab2s generated by the Ab1: Ab2α, Ab2β and Ab2γ antibodies. According to the network nomenclature,10 Ab2α binds to a region of Ab1 outside the paratope or combining site; its binding is not inhibited by cocaine. Thus, the identification of Ab2α antibodies was easily accomplished through inhibition assays using soluble cocaine. The Ab2β, the internal image of the antigen, recognizes the paratope or a discrete region within the antigen-combining site of the Ab1: as a consequence, its binding to Ab1 is inhibited by the antigen. Ab2γ, which binds a region of the Ab1 in very close proximity to the binding site of the Ab1, is much more difficult to distinguish from an Ab2β as it also interferes with the paratope function of the Ab1. Ab2γ partial binding to the paratope can be inhibited by soluble cocaine, thus resembling the binding of Ab2β. Table 3 shows the patterns of cross-reactivity of 20 mAb2 of the β or γ type derived from Ab1s specific for the K1 conjugate, and 11 derived from Ab1s specific for the K2 conjugate. Ultimately, Ab2β were distinguished from the Ab2γ antibodies by immunization of animals with the antibodies and consequent presence of an Ab1-like Ab3 response. Such response is elicited only by the Ab2β antibody, which contains the internal image of cocaine.
Table 3.
Reactivity of anti-idiotypic mAb2 (β or γ) antibodies for various anticocaine Ab1 antibodies
| Ab1 used to elicit Ab2β/γ | Ab2β/γ* Designation | Reactivity with Ab1† | ||
|---|---|---|---|---|
| K1–1 | K1–2 | K1–4 | ||
| K1–1 | K1–1: a,c,f,ll | + | + | + |
| K1–1: b,d,e,g,h,i,j,k | + | – | – | |
| K1–2 | K1–2: a,b | – | + | – |
| K1–4 | K1–4: a,b,c,d,e,ff | + | + | + |
| K2–1 | K2–2 | K2–3 | ||
| K2–1 | K2–1: a,b,c,d | + | – | – |
| K2–2 | K2–2: a,b | – | + | + |
| K2–3 | K2–3: a | – | + | + |
| K2–3: b,c,d,e | – | – | + |
mAb2 were identified by the designation of the mAb1 that elicited them followed by a letter (K1–1a, K1–1b, etc., K1–2a, K1–2b, etc.).
Each Ab2 was reacted on plates coated with the Ab1 that elicited it or with other Ab1s specific for the homologous conjugate. The Ab2s are grouped according to identical reactivity patterns.
Ab1-like Ab3 response
To date, of the 31 mAb2 antibodies characterized in Table 3, 17 were chosen to be used in the immunization of mice for the elicitation of an Ab1-like Ab3 response. These representative Ab2 antibodies were chosen based on their different cross-reacting characteristics because, at this time, it was unclear which characteristics could be attributed to an Ab2β antibody.
Of the 17 mAb2 antibodies tested, only four elicited an Ab1-like Ab3 immune response specific for cocaine, confirmed through competitive ELISA (Table 4). These four antibodies: K1–4a, K1–4b, K1–4c and K1–4e, were therefore identified as Ab2β antibodies containing the internal image of cocaine. Interestingly, all four of these Ab2β antibodies were elicited by the antibody K1–4 and all four displayed cross-reactivity with the other K1-specific antibodies (Table 3).
Table 4.
Selection of mAb2β by elicitation of an Ab1-like Ab3 response in BALB/c mice
| Ab1 used to elicit the Ab2s | Antigen: mAb2β or γ* | Abl-like Ab3 response† (serum titre) ‡ |
|---|---|---|
| K1–1 | K1–1a | – |
| K1–1b | – | |
| K1–1c | – | |
| K1–1d | – | |
| K1–2 | K1–2a | – |
| K1–4 | K1–4a | 800 |
| K1–4b | 3200 | |
| K1–4c | 3200 | |
| K1–4d | – | |
| K1–4e | 3200 | |
| K1–4ff | – | |
| K2–1 | K2–1a | – |
| K2–1b | – | |
| K2–2 | K2–2a | – |
| K2–2b | – | |
| K2–3 | K2–3a | – |
| K2–3b | – |
At least four BALB/c female mice were injected with each mAb2 in Freund's complete adjuvant (FCA) intraperitoneally (i.p.), followed by repeated injections with mAb2 in Freund's incomplete adjuvant (FIA) at 2-week intervals. Blood for serum titration was obtained from the retroorbital cavity under anesthesia.
Ab1-like Ab3 response was assayed by enzyme-linked immunosorbent assay (ELISA) on plates coated with the appropriate conjugate. All positive responses were inhibited by soluble cocaine.
Serum titre was defined as that dilution of sample giving an absorbance equal to 50% of the maximum absorbance of a positive control (pooled Ab1 sera).
Distribution of cocaine in Ab2β-vaccinated mice
Six BALB/c mice were immunized with K1–4c, K1–4e or a mixture of all four mAb2βs. Each received four injections; antibodies specific for cocaine were present in their serum 3 weeks after the first injection and increased as the immunization continued. A group of controls similarly injected with a mAb2γ did not show any anti-cocaine antibodies in their sera. Ten days after the last injection, the animals were injected i.p. with 5 mg/kg body weight of cocaine. Ten minutes later, the brains were obtained, extracted and analysed. A 10%, 33% and 36% mean reduction in the level of cocaine in the brain of mice injected with K1–4e, K1–4c and the mix, respectively, was obtained, over the level observed in the controls. The 36% reduction was statistically significant (P < 0·05); the 33% reduction was borderline (P = 0·056) (data not shown).
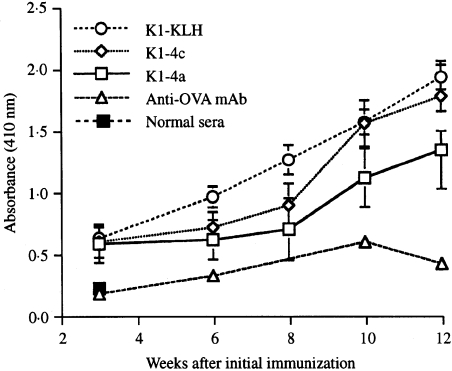
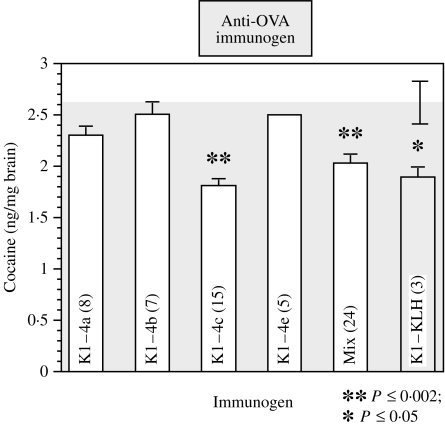
In a second, larger experiment, a group of BALB/c mice were immunized with one of the four mAb2β antibodies, a mixture of all four, the conjugate K1-KLH, or with an isotype-matched anti-ovalbumin (anti-OVA) mAb as control. Each mouse received six injections; the level of cocaine-specific antibodies in their serum was monitored after each injection. As the immunization progressed, increasing levels of anticocaine antibodies in the sera of two representative groups of mice injected with Ab2β (K1–4a and K1–4c) and of the group injected with the cocaine conjugate were observed (Fig. 2). The specificity of these responses was confirmed by inhibition assays using soluble cocaine. The percentage inhibition at the serum titre dilution for K1–4a, K1–4c and K1–KLH were 57, 94 and 89, respectively. The low reactivity of the anti-OVA mAb (Fig. 2) was not inhibited even by a high concentration of soluble cocaine. After the sixth injection, the animals were challenged as in the previous experiment and their brains analysed. As expected, the concentration of cocaine in the brains of mice immunized with the cocaine conjugate was significantly lower than that of the control mice (28·5% reduction). As shown in Fig. 3, highly significant reductions were also obtained in mice immunized with K1–4c or the mixture of the four Ab2β (31·5% and 22·8%, respectively), while the reduction was insignificant in mice immunized with the other three Ab2β (12·5%, 5% and 4·5%). Similar significant reductions in brain cocaine levels were obtained when the average concentration of cocaine, following challenge of normal unimmunized young mice, was used as control.
Figure 2.
Level of anticocaine antibodies in the sera of mice immunized with cocaine hapten precursor or monoclonal anti-idiotypic antibodies specific for Ab1 (mAb2β) or irrelevant antibody conjugated to keyhole limpet haemocyanin (KLH) in alum, expressed as mean absorbance (± SD) of samples at a 1 : 50 dilution. Each point represents eight mice. The mice were injected at 0, 3, 6, 8, 10 and 12 weeks. Beginning with the second injection, the mice were bled before each injection. Enzyme-linked immunosorbent assay (ELISA) plates were coated with K1–bovine serum albumin (BSA) and developed with enzyme-linked goat anti-mouse immunoglobulin.
Figure 3.
Average concentration of cocaine, following challenge, in the brains of male mice immunized with various monoclonal anti-idiotypic antibodies specific for Ab1β (mAb2β) (white bars) or with the cocaine conjugate (textured bar) were compared with the concentration found in the brain of mice immunized with anti-ovalbumin (anti-OVA), an irrelevant monoclonal antibody (grey area). Standard error bars are shown. Immunization consisted of one initial injection intraperitoneally (i.p.) with 100 μg of alum-flocculated antigen, followed by one injection of 50 μg of alum-flocculated antigen at 3 weeks and three similar injections at 2-week intervals. Equal amounts of each of the four mAb2β, for a total of 100 μg or 50 μg, were used for injection of the mix group. Ten days before cocaine challenge, the mice were given 50 μg of soluble antigen. The challenge consisted of 5 mg/kg body weight i.p. of cocaine in saline. The dosage of 5 mg/kg cocaine i.p. challenge was chosen because it resulted in an easily detectable peak by HPLC and it is well within the range of the doses used by humans. Ten minutes later the mice were killed by cervical dislocation, and the brain was removed, weighed and frozen. KLH, keyhole limpet haemocyanin.
Discussion
This study proposed:
To produce monoclonal anti-idiotypic Ab2βs (mAb2βs) by immunization of mice with anticocaine mAb1s;
To ascertain whether mice immunized with these mAb2βs produce an Ab1-like Ab3 response; and
To investigate the effects that such immunization may have on cocaine distribution after challenge.
Because the aim of this work was to use mAb1s as immunogens to produce ‘internal image’ mAb2βs, it was important that all features of the cocaine molecule be represented in the immunogen used in the production of mAb1. To achieve this, two cocaine conjugates (K1, K2), which present different sides of the cocaine molecule to the immune system, were synthesized. Both conjugates contained the SPDP spacer between the cocaine moiety and the protein carrier molecule. This spacer should place the haptenic groups at some distance from the carrier and thus avoid steric interference by the carrier with the binding of the appropriate B-cell receptor. One Ab1 specific for the conjugate K1 (K1–4) elicited four Ab2βs (K1–4a,b,c,e) molecules. The reactivity of K1–4 with the various metabolites of cocaine clearly showed that all parts of the cocaine molecule (the N-methyl group, the 2β methylester and the 3β benzoylester) are involved in binding with K1–4. The lack of reactivity of K1–4 with the conjugate K2 and norcocaine, neither of which possess the N-methyl group, reinforces the idea of an extensive interaction between K1–4 and the cocaine molecule. None of the tested Ab1s specific for the conjugate K2 elicited Ab2β. Although K2–2 and K2–3 bind cocaine with a higher affinity than K1–4, only the 2β methylester and the 3β benzoylester groups are involved in the binding; furthermore, their almost identical affinity for cocaine and norcocaine suggests a more limited area of interaction. It is tempting to hypothesize that an idiotype recognizing a greater area of the cocaine molecule, albeit with a modest strength of interaction, is more likely to elicit Ab2β than an idiotype recognizing a smaller area, albeit with a higher affinity. However, this hypothesis may be premature as K1–1, which showed similar characteristics to K1–4, did not elicit Ab2β; furthermore, only a few anti-idiotypic molecules derived from K2–2 and K2–3 were studied. Production and analysis of a greater number of idiotypes specific for both conjugates will provide a better understanding of the characteristics required for an Ab1 to elicit an Ab2β. K1–4 did not show reactivity with BE and would be unlikely to elicit internal images to BE. This is desirable for an Ab1, as BE, the major metabolite of cocaine in the blood, is not pharmacologically active and hence not likely to result in an efficient vaccine.
The reduction in brain cocaine levels observed in mice immunized with K1–4c was comparable to that seen in mice immunized with the cocaine conjugate K1–KLH, as was their serum level of anticocaine antibodies. The level of anticocaine antibodies induced by the other Ab2βs was lower than that elicited by the K1–KLH. The use of an immunizing mixture of mAb2βs would seem to be a preferable alternative if additional Ab2βs, which perform individually as well or better than the K1–4c, are obtained.
Vaccination is a novel approach to the problem of cocaine addiction. Unlike vaccination against infectious agents, the efficacy of a vaccine against cocaine would rely on the constant presence of circulating cocaine-specific antibodies, rather than on the ability of the body to produce an enhanced secondary immune response. Unfortunately, repeated exposure to soluble cocaine, taken by the user, would not elicit an enhanced secondary immune response. Therefore, an effective cocaine vaccine would need to consist of repeated immunizations.
Several independent studies using conventional vaccines have firmly established that the presence in the circulation of antibodies specific for cocaine significantly decreases and blunts the distribution of cocaine to the brain after challenge.2−4,6 As long as the level of cocaine in the brain remains under a certain threshold value, its pharmacological effect is greatly reduced. Through positron emission tomography, Volkow et al.28 determined that at least 47% of the dopamine reuptake transporters must be occupied for human subjects to perceive the effects of cocaine. Fox and others2,3,6 demonstrated that the decrease in cocaine in the brain of immunized mice does not represent a delay in the kinetics of distribution, but a true reduction.
This study establishes that it is possible to generate Ab2β molecules which mimic the cocaine molecule and to use these Ab2βs as immunogens instead of the cocaine conjugates. Anti-idiotypic vaccines have been examined for several years and have been proven successful in certain situations. In this case, the use of an anti-idiotypic vaccine would circumvent the problems associated with the instability of the cocaine molecule, an important consideration because an immune response to the metabolites of cocaine would be of little use.2 In addition, there may well be objections to the direct use of the drug as a vaccine, even if it is chemically altered by conjugation to the carrier. Therefore, the use of a harmless anti-idiotypic antibody as a surrogate immunogen is an alternative worth further exploration.
We are confident that additional effective Ab2βs can be produced, and that ways to increase the immunogenicity of the antibody molecule can be found.
Acknowledgments
We acknowledge the generous help of Dr John A. Katzenellenbogen in the production of the two cocaine haptens, and that of Dr Mark S. Kuhlenschmidt in the production of the cocaine conjugates and the determination of the levels of haptenation. The valuable help of Dr E. H. Greeley in editing this manuscript is also acknowledged. This work was supported by grants from the Research Board of the University of Illinois at Urbana and the National Institute of Drug Abuse, Washington, DC.
Glossary
Abbreviations
- BE
benzoylecgonine
- Ab1
idiotypic antibody specific for cocaine
- Ab2
anti-idiotypic antibody specific for Ab1
References
- 1.Gavin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- 2.Carrera MRA, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effect of cocaine by active immunization. Nature. 1995;378:727. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 3.Fox BS, Kantak K, Edwards M, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nature Med. 1996;2:1129. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger RH, Ettinger W, Harless W. Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharm Biochem Behav. 1997;58:215. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 5.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature. 1974;252:708. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 6.Borman S. Cocaine vaccine developed: antibodies block drug's psychoactivity. Chem Engineering News, Dec. 1995;18:6. [Google Scholar]
- 7.Tramontano A, Janola KD, Lerner RA. Catalytic antibodies. Science. 1986;234:1566. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 8.Chandrakumar NS, Carron CP, Meyer DM, et al. Phenylphosphonate monoester analogs of cocaine. Potential haptens for the generation of catalytic antibodies. Biorg Med Chem Lett. 1993;3:309. [Google Scholar]
- 9.Landry DW, Zhao K, Yang GX-Q, Glickman M, Georgiadis TM. Antibody-catalyzed degradation of cocaine. Science. 1993;259:1899. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 10.Jerne NK. Toward a network theory of the immune system. Ann Immunol (Inst Pasteur) 1974;125C:373. [PubMed] [Google Scholar]
- 11.Thanavala Y. Novel approaches to vaccine development against HBV. J Biotechnol. 1996;44:67. doi: 10.1016/0168-1656(95)00117-4. [DOI] [PubMed] [Google Scholar]
- 12.Deckert PM, Ballmaier M, Lang S, Deicher H, Schedel I. CD4-imitating human antibodies in HIV infection and anti-idiotypic vaccination. J Immunol. 1996;157:826. [PubMed] [Google Scholar]
- 13.Hutchins WA, Kieber-emmons T, Carlone GM, Westerink MA. Human immune response to a peptide mimic of Neisseria meningitidis serogroup C in hu-PBMC-SCD mice. Hybridoma. 1999;18:121. doi: 10.1089/hyb.1999.18.121. [DOI] [PubMed] [Google Scholar]
- 14.Whittum-hudson JA, An L, Saltzman WM, Prendergast RA, MacDonald AB. Oral immunization with anti-idiotypic antibody to the exoglycolipid antigen protects against experimental Chlamydia trachomatis infections. Nature Med. 1996;2:1116. doi: 10.1038/nm1096-1116. [DOI] [PubMed] [Google Scholar]
- 15.Tripathi PK, Qin H, Bhattacharya-chatterjee M, Ceriani RL, Foon KA, Chatterjee SK. Construction and characterization of a chimeric fusion protein consisting of an anti-idiotype antibody mimicking a breast cancer-associated antigen and the cytokine GM-CSF. Hybridoma. 1999;18:193. doi: 10.1089/hyb.1999.18.193. [DOI] [PubMed] [Google Scholar]
- 16.Field SK, Morrison DC. An anti-idiotype antibody which mimics the inner-core region of lipopolysaccharide protects mice against a lethal challenge with endotoxin. Infect Immun. 1994;62:3994. doi: 10.1128/iai.62.9.3994-3999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant SC, Kris MG, Houghton AN, Chapman PB. Long survival of patients with small cell lung cancer after adjuvant treatment with anti-idiotypic antibody BEC2 plus bacillus Calmette–Guerin. Clin Cancer Res. 1999;5:1319. [PubMed] [Google Scholar]
- 18.Reinartz S, Boerner H, Koehler S, von Reucker A, Schlebusch H, Wagner U. Evaluation of immunological responses in patients with ovarian cancer treated with the anti-idiotype vaccine ACA125 by determination of intracellular cytokines; a preliminary report. Hybridoma. 1999;18:41. doi: 10.1089/hyb.1999.18.41. [DOI] [PubMed] [Google Scholar]
- 19.Lewin AH, Gao Y, Abraham P, Boja JW, Kuhar MJ, Carroll FI. 2 Beta-substituted analogues of cocaine. Synthesis and inhibition of binding to the cocaine receptor. J Med Chem. 1992;35:135. doi: 10.1021/jm00079a017. [DOI] [PubMed] [Google Scholar]
- 20.Meltzer PC, Liang AY, Brownell A-L, Elmaleh DR, Madra BK. Substituted 3-phenyltropane analogs of cocaine: synthesis, inhibition of binding at cocaine recognition sites, and positron tomography imaging. J Med Chem. 1993;36:855. doi: 10.1021/jm00059a010. [DOI] [PubMed] [Google Scholar]
- 21.Oi VT, Herzenberg LA. Immunoglobulin-producing hybrid cell lines. In: Bhishell B, Shiigi S H, editors. Selected Methods in Cellular Immunology. San Francisco, CA: W. H. Freeman & Co.; 1980. pp. 303–354. [Google Scholar]
- 22.Muhumuza L, Segre D, Segre M. Antibodies with idiotypic and anti-idiotypic reactivity (epibodies) in conventional immune responses to dinitrophenylated carriers. Immunology. 1998;93:572. doi: 10.1046/j.1365-2567.1998.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller UW, Hawes CS, Jones WR. Monoclonal antibody production by hybridoma growth in Freund's adjuvant primed mice. J Immunol Methods. 1986;87:193. doi: 10.1016/0022-1759(86)90530-2. [DOI] [PubMed] [Google Scholar]
- 24.Reik LM, Maines SL, Ryan DE, Levin W, Bandiera S, Thomas PE. A simple, non-chromatographic purification procedure for monoclonal antibodies. J Immunol Methods. 1987;100:123. doi: 10.1016/0022-1759(87)90180-3. [DOI] [PubMed] [Google Scholar]
- 25.Friguet B, Chaffotte AF, Djaradi-ohaniance L, Golberg ME. Measurements of the true affinity constant in solution of antigen-antibody complexes by ELISA. J Immunol Methods. 1985;77:305. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 26.Nih . Current Protocols in Immunology. New York, NY: Wiley Interscience; 1999. [Google Scholar]
- 27.Whitlow M, Howard AJ, Wood JF, Voss EW, Hardman KD. 1.85A structure of anti-fluorescein 4-4-20 Fab. Protein Eng. 1995;8:749. doi: 10.1093/protein/8.8.749. [DOI] [PubMed] [Google Scholar]
- 28.Volkow N, Wang G, Fischman M, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]