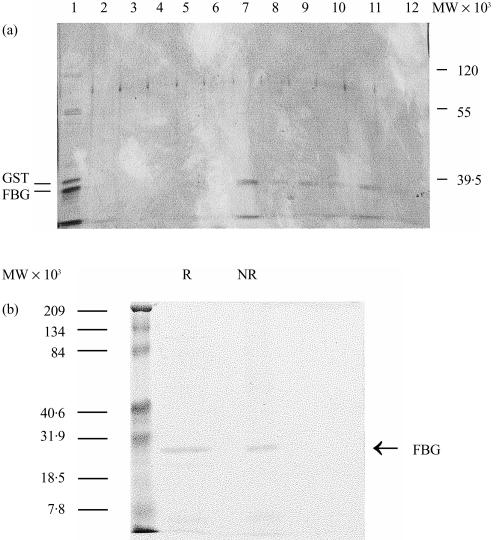
Figure 1.
Chracterization of M-ficolin fibrinogen-like (FBG) domain. (a) The FBG domain of M-ficolin was expressed as a glutathione-S-transferase (GST) fusion protein. The fusion protein bound to GSH–Sepharose was digested in gel with thrombin and the FBG domain released into the supernatant was collected and applied to an N-acetyl-d-glucosamine (GlcNAc)–Sepharose column. Unbound proteins, shown in lane 1, included predominantly the 24 000-molecular weight (MW) FBG domain and some contaminating GST (the 26 000-MW band). The resin was washed five times each with two bed volumes of phosphate-buffered saline (PBS) and then eluted with 0·1 m GlcNAc in the same buffer. The unbound proteins (lane 1), the washings (lanes 2–6) and the GlcNAc eluate (lanes 7–12) were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on a 12·5% (w/v) gel and visualized by silver-staining. (b) The purified FBG was examined by SDS–PAGE on a 12·5% (w/v) gel in the presence (R) or absence (NR) of 2-mercaptoethanol and the gel was stained with Coomassie Brilliant blue. The molecular weight standards are indicated on the left.